Betapace AF
Active Ingredient(s): SotalolFDA Approved: * February 22, 2000
Pharm Company: * BERLEX
Category: Irregular Heartbeat (Arrhythmia)
Sotalol, sold under the brand name Betapace among others, is a medication used to treat and prevent abnormal heart rhythms.[1] It is only recommended in those with significant abnormal heart rhythms due to potentially serious side effects.[1] Evidence does not support a decreased risk of death with long term use.[1] It is taken by mouth or injection into a vein.[1] Common side effects include a slow heart rate, chest pain, low bl... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List
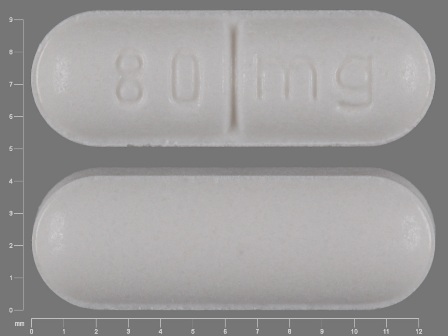
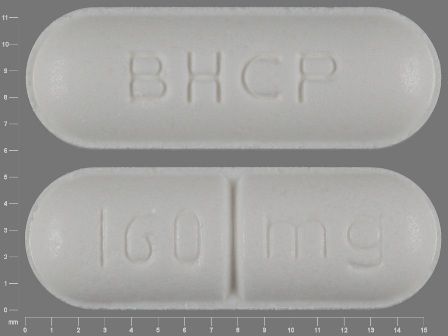




Popular Topics
Is it safe to take betapace opened up instead of taking the capsule? ## If your doctor or pharmacist have not said it wa...
My periods are due on the 28th of December. Doctor has advised me to take Regestrone tablets twice a day since the 24th ...
I heard about this product on the radio for a free trial, so before I called I decided to look into it a little more. I ...
I heard an ad for this on the radio today. I've never heard of it and just really wanted to know, what's in it a...
I got the free trial and saw no difference in my weight or energy levels. When I called to cancel and return the unused ...
I have been diagnosed with Fibromyalgia and Depression. I have been on many different meds and am feeling very frustrate...
I ordered it and also called the company, but I haven't seen the package yet, all tho it has been sometime now. I wa...
I have been treated with BV earlier this year, and my bf and I had contact 3 weeks ago. after a few days, I noticed my v...
I have been on anti-depressants since 1999 and would like to get off them altogether. I started with Paxil and after sev...
Completed 8 weeks of Harvoni. Still having diahhrea (daily) and very tired 4 months later (sleep 10+ hours per night &am...