0131-3268 : Venlafaxine 225 mg 24 Hr Extended Release Tablet
| NDC: | 0131-3268 |
| Labeler: | Schwarz Pharma Manufacturing, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Venlafaxine Hydrochloride Venlafaxine Hydrochloride |
| Dosage Form: | Oral Tablet, Extended Release |
| Application #: | NDA022104 |
| Rev. Date: |
Appearance:
| Markings: | OS304 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 11 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
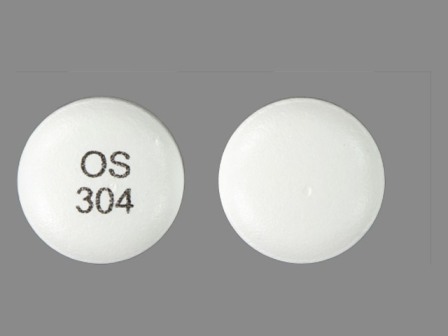
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0131-3268-32: 30 TABLET, EXTENDED RELEASE IN 1 BOTTLE (0131‑3268‑32)
- 0131-3268-46: 90 TABLET, EXTENDED RELEASE IN 1 BOTTLE (0131‑3268‑46)
Active Ingredients:
- Venlafaxine Hydrochloride
Dosage Strength:
- 225 mg
Inactive Ingredients:
- Mannitol
- Povidone
- Cellulose, Microcrystalline
- Polyethylene Glycol
- Silicon Dioxide
- Magnesium Stearate
- Hypromelloses
- Lactose
- Titanium Dioxide
- Triacetin
- Ferrosoferric Oxide
- Propylene Glycol
Pharmaceutical Classes:
- Norepinephrine Uptake Inhibitors [MoA]
- Serotonin and Norepinephrine Reuptake Inhibitor [EPC]
- Serotonin Uptake Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 0131-3265 Venlafaxine 37.5 mg 24 Hr Extended Release Tablet by Schwarz Pharma Manufacturing, Inc.
- 0131-3266 Venlafaxine 75 mg 24 Hr Extended Release Tablet by Schwarz Pharma Manufacturing, Inc.
- 0131-3267 Venlafaxine 150 mg 24 Hr Extended Release Tablet by Schwarz Pharma Manufacturing, Inc.
- 0093-0199 Venlafaxine 25 mg (As Venlafaxine Hydrochloride 28.3 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7380 Venlafaxine 37.5 mg (As Venlafaxine Hydrochloride 42.5 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7381 Venlafaxine 50 mg (As Venlafaxine Hydrochloride 56.6 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7382 Venlafaxine 75 mg (As Venlafaxine Hydrochloride 84.9 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7383 Venlafaxine 100 mg (As Venlafaxine Hydrochloride 113 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7384 Venlafaxine (As Venlafaxine Hydrochloride) 37.5 mg 24 Hr Extended Release Capsule by Teva Pharmaceuticals USA Inc
- 0093-7385 Venlafaxine (As Venlafaxine Hydrochloride) 75 mg 24 Hr Extended Release Capsule by Teva Pharmaceuticals USA Inc
- 0093-7386 Venlafaxine (As Venlafaxine Hydrochloride) 150 mg 24 Hr Extended Release Capsule by Teva Pharmaceuticals USA Inc
- 0093-9147 Venlafaxine Hydrochloride 25 mg Oral Tablet by Teva Pharmaceuticals USA, Inc.
- 0093-9148 Venlafaxine Hydrochloride 37.5 mg Oral Tablet by Teva Pharmaceuticals USA, Inc.
- 0093-9149 Venlafaxine Hydrochloride 50 mg Oral Tablet by Teva Pharmaceuticals USA, Inc.
- 0093-9157 Venlafaxine Hydrochloride 75 mg Oral Tablet by Teva Pharmaceuticals USA, Inc.
- 0093-9163 Venlafaxine Hydrochloride 100 mg Oral Tablet by Teva Pharmaceuticals USA, Inc.
- 0179-0107 Venlafaxine 25 mg (As Venlafaxine Hydrochloride 28.3 mg) Oral Tablet by Kaiser Foundation Hospitals
- 0179-0108 Venlafaxine 50 mg (As Venlafaxine Hydrochloride 56.6 mg) Oral Tablet by Kaiser Foundation Hospitals
- 0179-0109 Venlafaxine 100 mg (As Venlafaxine Hydrochloride 113 mg) Oral Tablet by Kaiser Foundation Hospitals
- 0179-0161 Venlafaxine Hydrochloride 37.5 mg Oral Capsule, Extended Release by Kaiser Foundation Hospitals
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0131-3267Next: 0131-5410 >
Related Discussions:
VENLAFAXINE HCL 75MG 24HR SA CAP
I have been on Cymbalta for RSD/CRPS pain and my VA doctor wants me to switch to VENLAFAXINE HCL 75MG 24HR SA CAP. She i... 8 replies
I have been on Cymbalta for RSD/CRPS pain and my VA doctor wants me to switch to VENLAFAXINE HCL 75MG 24HR SA CAP. She i... 8 replies
Venlafaxine XR and Dizziness/Vertigo
My doctor changed me from Effexor XR capsules 37.5 mg. to 37.5 mg Venlafaxine XR (white tablet). I am now having a lot o... 7 replies
My doctor changed me from Effexor XR capsules 37.5 mg. to 37.5 mg Venlafaxine XR (white tablet). I am now having a lot o... 7 replies
Venlafaxine Hcl 150 mg 24 HR SA Tab
I want to get off of venlafaxine HCL 150 mg 24 HR SA Tab. There is no score line. Can I cut it in half and take just hal... 5 replies
I want to get off of venlafaxine HCL 150 mg 24 HR SA Tab. There is no score line. Can I cut it in half and take just hal... 5 replies
Venlafaxine HCL ER manufactured by Aurobino contact FDA
I have been taking generic Venlafaxine from Tega Pharma for the past 5 years and have had no issues. My pharmacy is now ... 5 replies
I have been taking generic Venlafaxine from Tega Pharma for the past 5 years and have had no issues. My pharmacy is now ... 5 replies
venlafaxine 75mg generic for effexor
i have taken effexof for years and i have never seen any like the one i have now. it is pale yellow round tablet smooth ... 4 replies
i have taken effexof for years and i have never seen any like the one i have now. it is pale yellow round tablet smooth ... 4 replies
Venlafaxine Er 37 5
Dr give me Venlafaxine ER 37.5 I took 1 pill a day after a week increase to 2 pills a day.. well I finish the medication... 3 replies
Dr give me Venlafaxine ER 37.5 I took 1 pill a day after a week increase to 2 pills a day.. well I finish the medication... 3 replies
Venlafaxine XR coverage changes
I was just informed by my pharmacy today today that my insurance will no longer allowe me to take Venlafaxine XR 150mg t... 2 replies
I was just informed by my pharmacy today today that my insurance will no longer allowe me to take Venlafaxine XR 150mg t... 2 replies
Venlafaxine Hcl 37 5 Mg Tablet
my hematology oncologist prescribe this to me for "hot flashes". I had breast cancer and a total hysterectomy an... 2 replies
my hematology oncologist prescribe this to me for "hot flashes". I had breast cancer and a total hysterectomy an... 2 replies
Venlafaxine Er 37 5 TESTS REQUIRED
I am taking Venlafaxine Er 37 5 for last one month, please guide what are the tests which can be done along with taking ... 2 replies
I am taking Venlafaxine Er 37 5 for last one month, please guide what are the tests which can be done along with taking ... 2 replies
Venlafaxine what are best brands.
Hi I've been on venlafaxine for years the amneal brand. Well now they have stopped making it. Can anyone recommend a... 1 reply
Hi I've been on venlafaxine for years the amneal brand. Well now they have stopped making it. Can anyone recommend a... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.