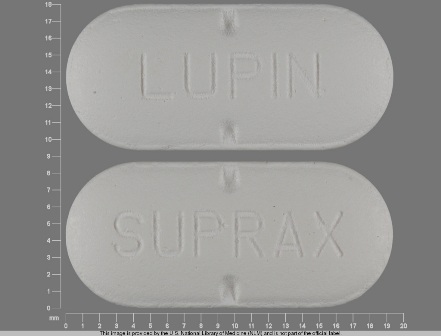
Suprax
Active Ingredient(s): CefiximeFDA Approved: * April 28, 1989
Pharm Company: * LEDERLE
Category: Antibiotics
Cefixime, sold under the brand name Suprax among others, is an antibiotic medication used to treat a number of bacterial infections.[4] These infections include otitis media, strep throat, pneumonia, urinary tract infections, gonorrhea, and Lyme disease.[4] For gonorrhea typically only one dose is required.[6] In the United States it is a second-line treatment to ceftriaxone for gonorrhea.[4] It is taken by mouth.[4&#... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List
Popular Topics
cefixime tergecef vs cefixime suprax
Hi. I just wanted to ask if the cefixime tergecef and cefixime suprax are the same in function and both if can cure gono...