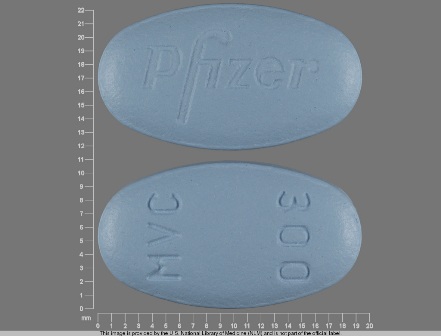
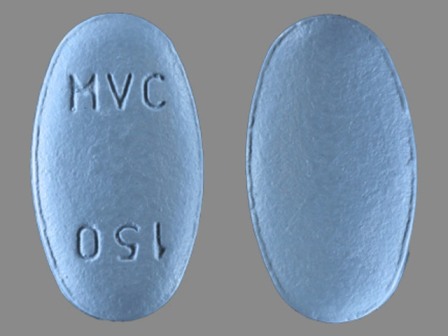
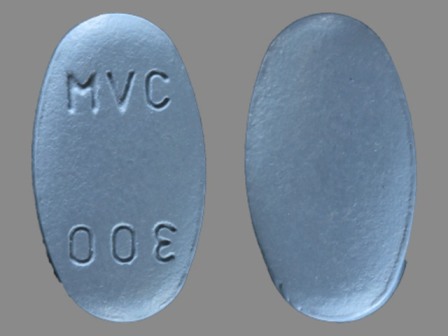
Selzentry
Active Ingredient(s): MaravirocFDA Approved: * August 6, 2007
Pharm Company: * PFIZER INC
Category: HIV / AIDS
Maraviroc, sold under the brand names Selzentry (US) and Celsentri (EU), is an antiretroviral drug in the CCR5 receptor antagonist class used in the treatment of HIV infection. It is also classed as an entry inhibitor. It also appeared to reduce graft-versus-host disease in patients treated with allogeneic bone marrow transplantation for leukemia, in a Phase I/II study.[5][6] Contents 1 Medical uses 2 Side effects 3 Mechanism of action 4 History 5 Names 6 See... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List