Risedronate
FDA Approved: * October 5, 2007Pharm Company: * TEVA PHARMS
Category: Osteoporosis
Risedronic acid, often used as its sodium salt risedronate sodium, is a bisphosphonate. It slows down the cells which break down bone. It's used to treat or prevent osteoporosis, and treat Paget's disease of bone. It is taken by mouth. It was patented in 1984 and approved for medical use in 1998.[1] Contents 1 Pharmacology 2 Society and culture 2.1 Brand names 2.2 Controversies 3 References 4 External links Pharmacology Relative potency[2] Bisphosp... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.6 Discussions
Dosage List
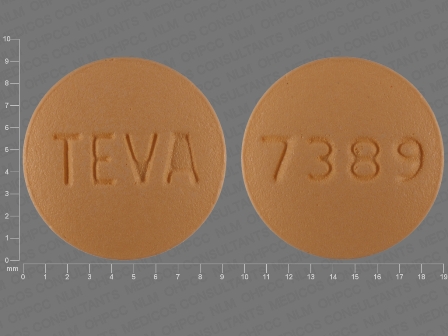
Risedronate Sodium 35 mg Oral Tablet, Film Coated
NDC: 0093-3098
Labeler:
Teva Pharmaceuticals USA Inc
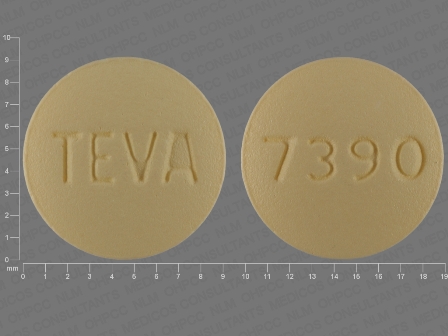
Risedronate Sodium 5 mg Oral Tablet, Film Coated
NDC: 0093-3099
Labeler:
Teva Pharmaceuticals USA Inc
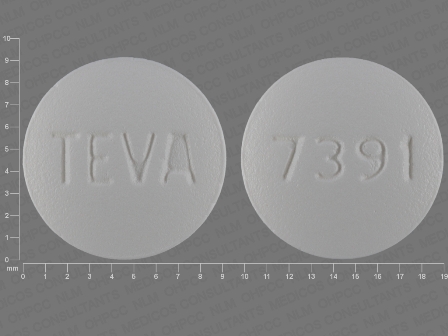
Risedronate Sodium 30 mg Oral Tablet, Film Coated
NDC: 0093-3100
Labeler:
Teva Pharmaceuticals USA Inc

Risedronate Sodium 35 mg Oral Tablet, Delayed Release
NDC: 0093-5509
Labeler:
Teva Pharmaceuticals USA Inc

Risedronate Sodium 150 mg Oral Tablet, Film Coated
NDC: 0093-7771
Labeler:
Teva Pharmaceuticals USA, Inc.
Risedronate Sodium 30 mg Oral Tablet, Film Coated
NDC: 0378-4114
Labeler:
Mylan Pharmaceuticals Inc.
Risedronate Sodium 150 mg Oral Tablet, Film Coated
NDC: 0378-4150
Labeler:
Mylan Pharmaceuticals Inc.
Risedronate Sodium 35 mg Oral Tablet, Film Coated
NDC: 0378-4714
Labeler:
Mylan Pharmaceuticals Inc.