What Does Taro Phn 100 Used For
16 Topics Found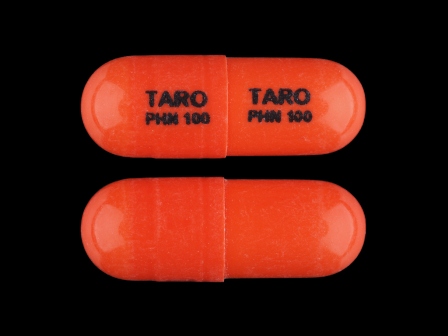
- NDC: *
- 51672-4111
- Imprint:
- TARO PHN 100
- Color:
- Orange
- Shape:
- Capsule
- Size:
- 16 mm
- Segments:
- 1
- Drug:
- Phenytoin
- Labeler: *
- Taro Pharmaceuticals U.S.a., Inc.
- Category:
- Anticonvulsant
Source: US National Library of Medicine (NLM)
This generic was given to me when I refilled my RX that had been Mylan 1560 white/lavender capsule. I haven't felt good, blahs, no energy, headache which I finally realized it started around the time I started taking the above medication. ## Hello, Deanna! How are you? I'm sorry about the way you're feeling. There can be slight fluctuations from one generic to another, so this one may have a slightly lower or higher dosage than the prior one. The difference is usually insignificant and your body will usually adjust in about a week or so. Have things improve at all, yet? How are you feeling?
Dph Sodium 100 mg Extended Release Capsule by Taro Pharmaceuticals U.S.a., Inc. ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 51672-4111-0, 51672-4111-1, 51672-4111-3, 51672-4111-6 ## Active Ingredients: Phenytoin Sodium
Dph Sodium 100 mg Extended Release Capsule by Cardinal Health ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 55154-3027-3, 55154-3027-7, 55154-3027-9 ## Active Ingredients: Phenytoin Sodium
Dph Sodium 100 mg Extended Release Capsule by Remedyrepack Inc. ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 52125-160-02 ## Active Ingredients: Phenytoin Sodium
Dph Sodium 100 mg Extended Release Capsule by Remedyrepack Inc. ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 52125-685-02 ## Active Ingredients: Phenytoin Sodium
Dph Sodium 100 mg Extended Release Capsule by Rebel Distributors Corp ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 21695-167-30, 21695-167-72, 21695-167-90 ## Active Ingredients: Phenytoin Sodium
Dph Sodium 100 mg Extended Release Capsule by Physicians Total Care, Inc. ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 54868-5534-0, 54868-5534-1, 54868-5534-2, 54868-5534-3, 54868-5534-4 ## Active Ingredients: Phenytoin Sodium
Dph Sodium 100 mg Extended Release Capsule by Contract Pharmacy Services-pa ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 67046-585-30, 67046-585-60 ## Active Ingredients: Phenytoin Sodium
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Remedyrepack Inc. ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 70518-0107-0 ## Active Ingredients: Phenytoin Sodium
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Remedyrepack Inc. ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 70518-2293-0, 70518-2293-1 ## Active Ingredients: Phenytoin Sodium
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Tya Pharmaceuticals ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 64725-4111-1 ## Active Ingredients: Phenytoin Sodium
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by A-s Medication Solutions ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 50090-3499-0 ## Active Ingredients: Phenytoin Sodium
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by A-s Medication Solutions ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 50090-3539-0, 50090-3539-1 ## Active Ingredients: Phenytoin Sodium
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Clinical Solutions Wholesale, LLC ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 58118-4111-8 ## Active Ingredients: Phenytoin Sodium
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Ncs Healthcare of Ky, Inc Dba Vangard Labs ## Markings: TARO;PHN;100 ## Shape: Capsule ## Color: Orange ## Package Codes: 0615-8020-30, 0615-8020-39 ## Active Ingredients: Phenytoin Sodium