Topiramate - NDC Database
513 records found
Page: 1 Start a Discussion
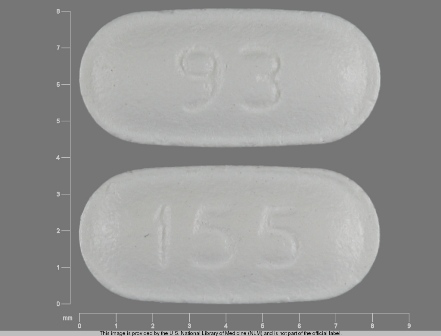
0093-0155 Mar 31, 2009
Topiramate 25 mg Oral Tablet by Teva Pharmaceuticals USA Inc
0093-7219 Mar 31, 2009
Topiramate 100 mg Oral Tablet by Teva Pharmaceuticals USA Inc
0093-7220 Mar 31, 2009
Topiramate 200 mg Oral Tablet by Teva Pharmaceuticals USA Inc
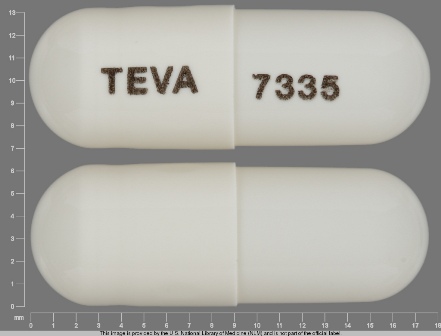
0093-7335 Apr 17, 2009
Topiramate 15 mg Oral Capsule by Teva Pharmaceuticals USA Inc
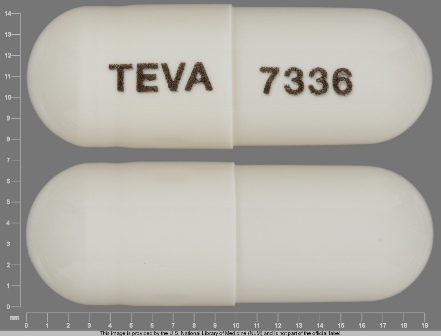
0093-7336 Apr 17, 2009
Topiramate 25 mg Oral Capsule by Teva Pharmaceuticals USA Inc
0093-7540 Mar 31, 2009
Topiramate 50 mg Oral Tablet by Teva Pharmaceuticals USA Inc
0378-6101 Dec 12, 2012
Topiramate 25 mg Oral Tablet by Mylan Pharmaceuticals Inc.
0378-6102 Dec 12, 2012
Topiramate 50 mg Oral Tablet by Mylan Pharmaceuticals Inc.
0378-6103 Dec 12, 2012
Topiramate 100 mg Oral Tablet by Mylan Pharmaceuticals Inc.
0378-6105 Dec 12, 2012
Topiramate 200 mg Oral Tablet by Mylan Pharmaceuticals Inc.
16252-569 Apr 15, 2009
Topiramate 25 mg Oral Capsule by Cobalt Laboratories
31722-278 Jan 01, 2011
Topiramate 25 mg Oral Tablet by Camber Pharmaceuticals
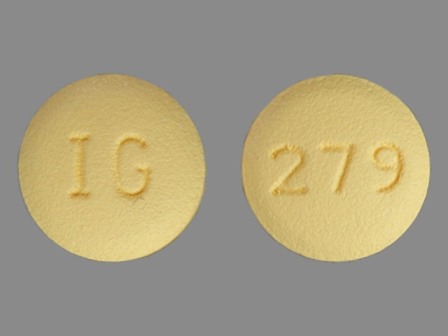
31722-279 Jan 01, 2011
Topiramate 50 mg Oral Tablet by Camber Pharmaceuticals
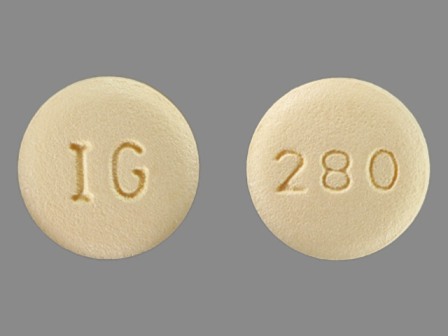
31722-280 Jan 01, 2011
Topiramate 100 mg Oral Tablet by Camber Pharmaceuticals
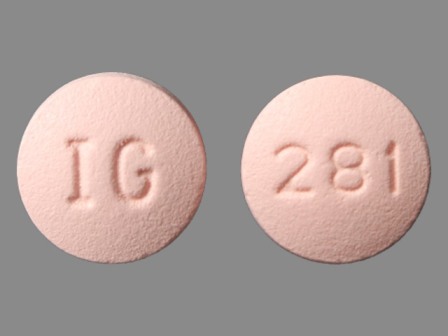
31722-281 Jan 01, 2011
Topiramate 200 mg Oral Tablet by Camber Pharmaceuticals
60429-769 Jul 08, 2011
Topiramate 25 mg Oral Tablet by Golden State Medical Supply, Inc.
60429-770 Jul 08, 2011
Topiramate 50 mg Oral Tablet by Golden State Medical Supply, Inc.
60429-771 Jul 08, 2011
Topiramate 100 mg Oral Tablet by Golden State Medical Supply, Inc.
60429-772 Jul 08, 2011
Topiramate 200 mg Oral Tablet by Golden State Medical Supply, Inc.
60505-2760 Mar 27, 2009
Topiramate 25 mg Oral Tablet by Apotex Corp
Page: 1 Start a Discussion
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .