1,150 records found
Page: 1 Start a Discussion
0004-6203 Sep 04, 1980
Anaprox Ds 550 mg Oral Tablet by Genentech, Inc.
0004-6415 Oct 14, 1994
Naprosyn 375 mg Enteric Coated Tablet by Genentech, Inc.
0004-6416 Oct 14, 1994
Naprosyn 500 mg Enteric Coated Tablet by Genentech, Inc.
0093-0147 Dec 22, 1993
Naproxen 250 mg Oral Tablet by Teva Pharmaceuticals USA Inc
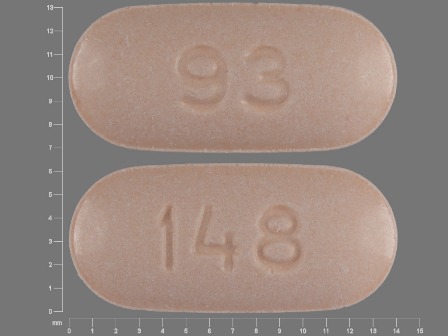
0093-0148 Dec 22, 1993
Naproxen 375 mg Oral Tablet by Teva Pharmaceuticals USA Inc
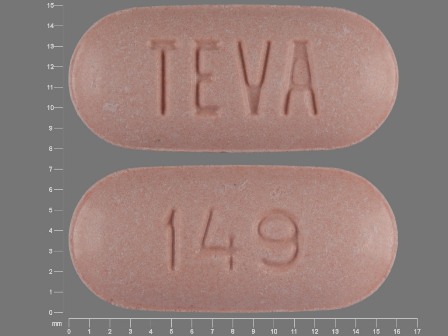
0093-0149 Dec 22, 1993
Naproxen 500 mg Oral Tablet by Teva Pharmaceuticals USA Inc
0093-0536 Dec 22, 1993
Naproxen Sodium 275 mg (Naproxen 250 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
0093-0537 Dec 22, 1993
Naproxen Sodium 550 mg (As Naproxen 500 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
0093-1005 Jul 31, 1998
Naproxen 375 mg (As Naproxen Sodium 413 mg) Enteric Coated Tablet by Teva Pharmaceuticals USA Inc
0093-1006 Jul 29, 1998
Naproxen 500 mg Enteric Coated Tablet by Teva Pharmaceuticals USA Inc
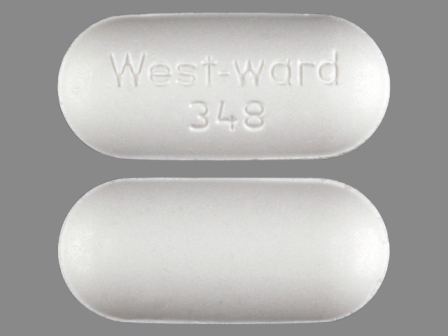
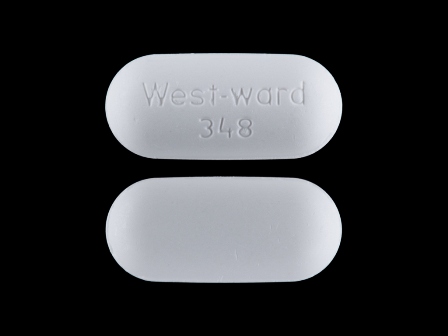
0143-1348 Jan 14, 2004
Naproxen 500 mg Oral Tablet by West-ward Pharmaceutical Corp
0143-9908 May 14, 1996
Naproxen Sodium 550 mg (As Naproxen 500 mg) Oral Tablet by West-ward Pharmaceutical Corp
0143-9916 Feb 18, 1998
Naproxen Sodium 275 mg (Naproxen 250 mg) Oral Tablet by West-ward Pharmaceutical Corp
0378-0451 Mar 01, 2010
Naproxen 500 mg Oral Tablet by Mylan Pharmaceuticals Inc.
0904-5591 Apr 01, 2004
Naproxen 500 mg Oral Tablet by Major Pharmaceuticals
0904-6069 Aug 10, 2009
Naproxen 250 mg Oral Tablet by Major Pharmaceuticals
27854-555 Jul 20, 2020
Belmora Flanax 220 mg Oral Tablet by Belmora LLC
31722-338 Jan 23, 2012
Naproxen 375 mg (As Naproxen Sodium 413 mg) Enteric Coated Tablet by Camber Pharmaceuticals
31722-339 Jan 23, 2012
Naproxen 500 mg Enteric Coated Tablet by Camber Pharmaceuticals
31722-340 Jan 23, 2012
Naproxen 250 mg Oral Tablet by Camber Pharmaceuticals
Page: 1 Start a Discussion
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .