Mirtazapine - NDC Database (Page 2)
324 records found
Start a Discussion
43353-535 Aug 17, 2009
Mirtazapine 15 mg Oral Tablet by Aphena Pharma Solutions - Tennessee, Inc.
43353-536 Aug 17, 2009
Mirtazapine 30 mg Oral Tablet by Aphena Pharma Solutions - Tennessee, Inc.
43353-614 Aug 17, 2009
Mirtazapine 45 mg Oral Tablet by Aphena Pharma Solutions - Tennessee, Inc.
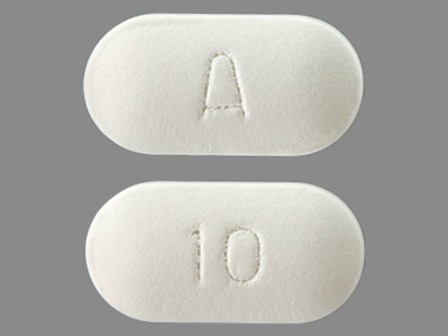
50090-3083 Jan 29, 2003
Mirtazapine 30 mg Oral Tablet, Film Coated by A-s Medication Solutions
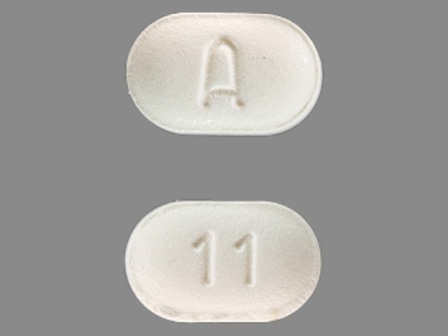
51079-086 Nov 08, 2011
Mirtazapine 15 mg Oral Tablet by Udl Laboratories, Inc.
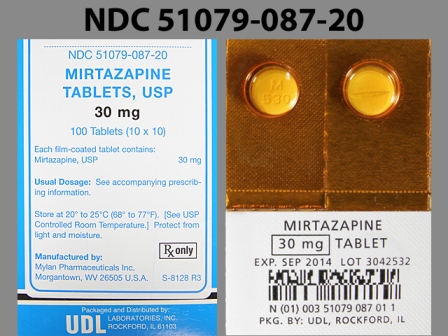
51079-087 Nov 09, 2011
Mirtazapine 30 mg Oral Tablet by Udl Laboratories, Inc.
55154-7141 Oct 31, 2005
Mirtazapine 30 mg Oral Tablet, Film Coated by Cardinal Health
57664-499 Apr 22, 2004
Mirtazapine 15 mg Oral Tablet by Caraco Pharmaceutical Laboratories, Ltd.
57664-500 Apr 22, 2004
Mirtazapine 30 mg Oral Tablet by Caraco Pharmaceutical Laboratories, Ltd.
59762-1415 Oct 22, 2004
Mirtazapine 7.5 mg Oral Tablet by Greenstone LLC
59762-1416 Oct 22, 2004
Mirtazapine 15 mg Oral Tablet by Greenstone LLC
59762-1417 Oct 22, 2004
Mirtazapine 30 mg Oral Tablet by Greenstone LLC
60505-0247 Aug 22, 2007
Mirtazapine 15 mg Oral Tablet by Apotex Corp.
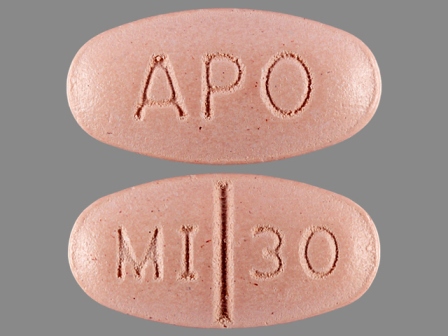
60505-0248 Aug 22, 2007
Mirtazapine 30 mg Oral Tablet by Apotex Corp.
60505-0249 Aug 22, 2007
Mirtazapine 45 mg Oral Tablet by Apotex Corp.
65862-003 Oct 22, 2004
Mirtazapine 30 mg Oral Tablet by Aurobindo Pharma Limited
65862-031 Oct 22, 2004
Mirtazapine 15 mg Oral Tablet by Aurobindo Pharma Limited
65862-032 Oct 22, 2004
Mirtazapine 45 mg Oral Tablet by Aurobindo Pharma Limited
66993-709 Jan 12, 2001
Mirtazapine 15 mg Disintegrating Tablet by Prasco Laboratories
66993-711 Jan 12, 2001
Mirtazapine 30 mg Disintegrating Tablet by Prasco Laboratories
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .