Levonorgestrel - NDC Database
85 records found
Page: 1 Start a Discussion
51285-103 Oct 28, 2014
Aftera 1.5 mg Oral Tablet by Teva Women's Health, Inc.
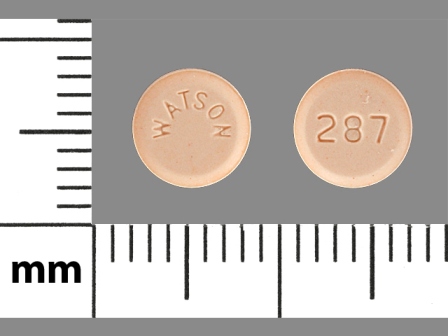
52544-287 Jul 16, 2012
Levonorgestrel 1.5 mg Oral Tablet by Watson Pharma, Inc.
51285-100 Feb 17, 2014
Take Action 1.5 mg/1 Oral Tablet by Teva Women's Health, Inc.
52544-065 May 12, 2014
Next Choice One Dose 1.5 mg Oral Tablet by Watson Pharma, Inc.
68071-1642 May 12, 2014
Next Choice One Dose 1.5 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
68071-4126 Feb 17, 2014
Take Action 1.5 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
69536-103 May 10, 2018
Aftera 1.5 mg Oral Tablet by Foundation Consumer Healthcare LLC
69536-133 Jun 15, 2019
Next Choice One Dose 1.5 mg Oral Tablet by Foundation Consumer Healthcare LLC
69536-200 May 10, 2018
Take Action 1.5 mg Oral Tablet by Foundation Consumer Healthcare LLC
0023-5858 May 31, 2016
Liletta 52 mg Intrauterine Intrauterine Device by Allergan, Inc.
0113-2003 Jan 29, 2016
Option 2 1.5 mg Oral Tablet by L. Perrigo Company
0536-1142 Nov 01, 2017
Levonorgestrel 1.5 mg Oral Tablet by Rugby Laboratories
0536-1391 Aug 01, 2023
Emergency Contraceptive 1.5 mg Oral Tablet by Rugby Laboratories, Inc.
11509-0067 Feb 19, 2018
Preventeza 1.5 mg Oral Tablet by Combe Incorporated
16714-809 Mar 11, 2016
New Day 1.5 mg Oral Tablet by Northstar Rx LLC
21695-443 Jul 21, 2009
Next Choice (Levonorgestrel 0.75 mg) Pack by Rebel Distributors Corp.
40032-620 Feb 22, 2013
Levonorgestrel 1.5 mg Oral Tablet by Novel Laboratories, Inc.
40032-622 Feb 15, 2013
Levonorgestrel 1.5 mg/1 Oral Tablet by Novel Laboratories, Inc.
40051-018 May 01, 2016
Levonorgestrel 1.5 mg Oral Tablet by Lotus Pharmaceutical Co., Ltd. Nantou Plant
40051-054 Sep 01, 2020
Levonorgestrel .75 mg Oral Tablet by Lotus Pharmaceutical Co., Ltd. Nantou Plant
Page: 1 Start a Discussion
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .