Galantamine - NDC Database (Page 2)

Galantamine 8 mg (As Galantamine Hydrobromide 10.253 mg) Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
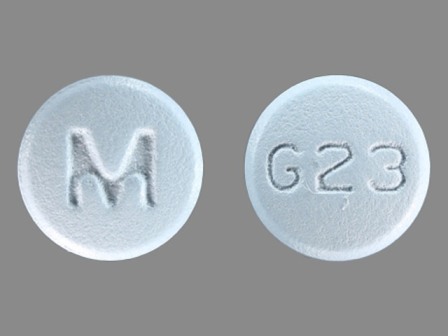
Galantamine 12 mg (As Galantamine Hydrobromide 15.379 mg) Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
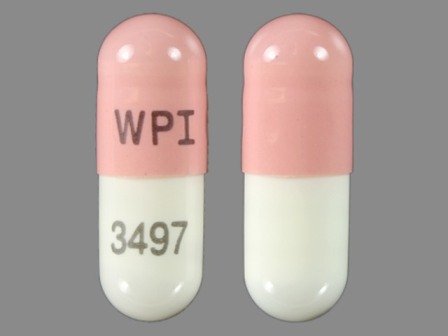
Galantamine Hydrobromide 16 mg Oral Capsule, Extended Release by Aphena Pharma Solutions - Tennessee, LLC

Galantamine 4 mg (As Galantamine Hydrobromide 5.126 mg) Oral Tablet by Mylan Institutional Inc.

Galantamine 8 mg (As Galantamine Hydrobromide 10.253 mg) Oral Tablet by Mylan Institutional Inc.

Galantamine 12 mg (As Galantamine Hydrobromide 15.379 mg) Oral Tablet by Mylan Institutional Inc.

Galantamine 4 mg (As Galantamine Hydrobromide 5.126 mg) Oral Tablet by Greenstone LLC

Galantamine 4 mg Oral Tablet, Film Coated by Mckesson Packaging Services a Business Unit of Mckesson Corporation

Galantamine 4 mg (As Galantamine Hydrobromide 5.126 mg) Oral Tablet by Aurobindo Pharma Limited

Galantamine 4 mg (As Galantamine Hydrobromide 5.126 mg) Oral Tablet by American Health Packaging

Galantamine Hydrobromide 4 mg Oral Tablet, Film Coated by Avera Mckennan Hospital
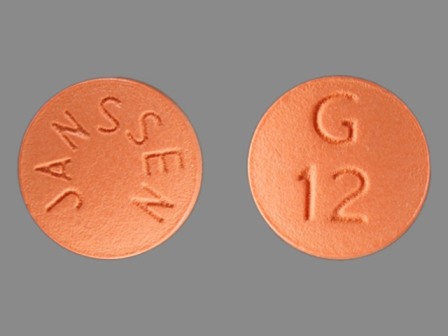
Galantamine Hydrobromide 12 mg Oral Tablet, Film Coated by Avera Mckennan Hospital
0054-0137 Aug 14, 2009
Galantamine Hydrobromide Oral Solution 4 mg/ml Oral Solution by Roxane Laboratories, Inc
Galantamine Hydrobromide Oral Solution 4 mg/ml Oral Solution by Roxane Laboratories, Inc
0115-1120 May 27, 2009
Galantamine Hydrobromide 8 mg 24 Hr Extended Release Capsule by Global Pharmaceuticals, Division of Impax Laboratories Inc.
Galantamine Hydrobromide 8 mg 24 Hr Extended Release Capsule by Global Pharmaceuticals, Division of Impax Laboratories Inc.
0115-1121 May 27, 2009
Galantamine Hydrobromide 16 mg 24 Hr Extended Release Capsule by Global Pharmaceuticals, Division of Impax Laboratories Inc.
Galantamine Hydrobromide 16 mg 24 Hr Extended Release Capsule by Global Pharmaceuticals, Division of Impax Laboratories Inc.
0115-1122 May 27, 2009
Galantamine Hydrobromide 24 mg 24 Hr Extended Release Capsule by Global Pharmaceuticals, Division of Impax Laboratories Inc.
Galantamine Hydrobromide 24 mg 24 Hr Extended Release Capsule by Global Pharmaceuticals, Division of Impax Laboratories Inc.
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.