Buprenorphine - NDC Database
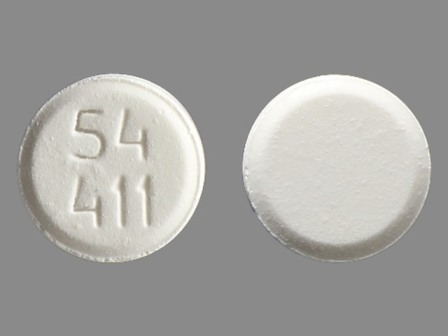
Buprenorphine 8 mg (As Buprenorphine Hydrochloride 8.64 mg) Sublingual Tablet by Roxane Laboratories, Inc

Buprenorphine 2 mg (As Buprenorphine Hydrochloride 2.16 mg) Sublingual Tablet by Teva Pharmaceuticals USA Inc

Buprenorphine 8 mg (As Buprenorphine Hydrochloride 8.64 mg) Sublingual Tablet by Teva Pharmaceuticals USA Inc

Buprenorphine 2 mg (As Buprenorphine Hydrochloride 2.16 mg) Sublingual Tablet by Remedyrepack Inc.

Buprenorphine Hcl 8 mg Sublingual Tablet by Lake Erie Medical Dba Quality Care Products LLC

Buprenorphine Hcl 8 mg Sublingual Tablet by Clinical Solutions Wholesale, LLC
0054-0176 Oct 08, 2009
Buprenorphine 2 mg (As Buprenorphine Hydrochloride 2.16 mg) Sublingual Tablet by Roxane Laboratories, Inc
Buprenorphine 2 mg (As Buprenorphine Hydrochloride 2.16 mg) Sublingual Tablet by Roxane Laboratories, Inc
0093-3239 Dec 27, 2021
Buprenorphine 7.5 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
Buprenorphine 7.5 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
0093-3600 May 30, 2017
Buprenorphine 5 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
Buprenorphine 5 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
0093-3601 May 30, 2017
Buprenorphine 10 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
Buprenorphine 10 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
0093-3602 May 30, 2017
Buprenorphine 15 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
Buprenorphine 15 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
0093-3603 May 30, 2017
Buprenorphine 20 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
Buprenorphine 20 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
0093-3656 Nov 26, 2018
Buprenorphine 5 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
Buprenorphine 5 ug/H Transdermal Patch, Extended Release by Teva Pharmaceuticals USA, Inc.
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.