51079-584 : Nitrofurantoin 50 mg Oral Capsule
| NDC: | 51079-584 |
| Labeler: | Udl Laboratories, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Nitrofurantoin (Macrocrystals) Nitrofurantoin (Macrocrystals) |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA074967 |
| Rev. Date: |
Appearance:
| Markings: | MYLAN;1650 |
| Shapes: |
Capsule |
| Colors: |
 Brown / Brown /
 Brown Brown |
| Size (mm): | 12 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
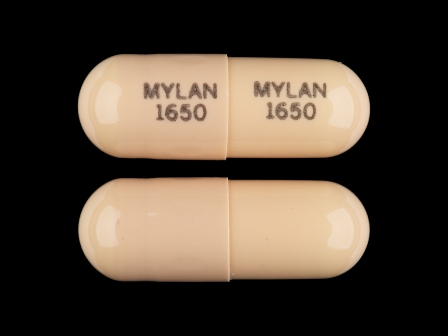
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 51079-584-20: 100 BLISTER PACK IN 1 BOX, UNIT‑DOSE (51079‑584‑20) > 1 CAPSULE IN 1 BLISTER PACK (51079‑584‑01)
Active Ingredients:
- Nitrofurantoin
Dosage Strength:
- 50 mg
Inactive Ingredients:
- Ferrosoferric Oxide
- Starch, Corn
- D&c Yellow No. 10
- Fd&c Blue No. 1
- Fd&c Blue No. 2
- Fd&c Red No. 40
- Gelatin
- Lactose Monohydrate
- Magnesium Stearate
- Ferric Oxide Red
- Silicon Dioxide
- Sodium Lauryl Sulfate
- Titanium Dioxide
- Ferric Oxide Yellow
Pharmaceutical Classes:
- Nitrofuran Antibacterial [EPC]
- Nitrofurans [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 51079-585 Nitrofurantoin 100 mg (Nitrofurantoin Macrocrystals 25 mg / Nitrofurantoin Monohydrate 75 mg) Oral Capsule by Udl Laboratories, Inc.
- 0378-1650 Nitrofurantoin 50 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-1700 Nitrofurantoin 100 mg (Nitrofurantoin Macrocrystals 25 mg / Nitrofurantoin Monohydrate 75 mg) Oral Capsule by Mylan Pharmaceuticals Inc.
- 35356-681 Nitrofurantoin 100 mg (Nitrofurantoin Macrocrystals 25 mg / Nitrofurantoin Monohydrate 75 mg) Oral Capsule by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
- 54569-0181 Nitrofurantoin 50 mg Oral Capsule by A-s Medication Solutions LLC
- 55289-186 Nitrofurantoin 50 mg Oral Capsule by Pd-rx Pharmaceuticals, Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 51079-582Next: 51079-585 >
Related Discussions:
Nitrofurantoin mono mac side effects
I am taking this medication for a UTI. I have been on it for a day and a half. This morning I was suddenly wakened by a ... 20 replies
I am taking this medication for a UTI. I have been on it for a day and a half. This morning I was suddenly wakened by a ... 20 replies
Nitrofurantoin mono/Mac 100mg side effects
After taking this medication for three days, I did not experience any side effects except for intense headaches right af... 7 replies
After taking this medication for three days, I did not experience any side effects except for intense headaches right af... 7 replies
NITROFURANTOIN AND BLOOD PRESSURE
I have been taking Nitrofurantoin 100mg, one per day for 2 months. Since taking it have had bad headaches, lightheadedne... 7 replies
I have been taking Nitrofurantoin 100mg, one per day for 2 months. Since taking it have had bad headaches, lightheadedne... 7 replies
Nitrofurantoin Mono Mac bad reaction
I am 15 weeks pregnant I was put on this medication for a UTI 2 capsules a day for 7 days I took 3 doses and didn't ... 7 replies
I am 15 weeks pregnant I was put on this medication for a UTI 2 capsules a day for 7 days I took 3 doses and didn't ... 7 replies
nitrofurantoin monohydrate
black and yellow with the word watson on the black part its a capsul ## it was prescribed to me for a urine infection th... 6 replies
black and yellow with the word watson on the black part its a capsul ## it was prescribed to me for a urine infection th... 6 replies
nitrofurantoin side effects
I am 76 years old. I was prescribed Nitrofurantoin 50 MG twice a day for a UTI. On the 11th day I developed a red rash a... 5 replies
I am 76 years old. I was prescribed Nitrofurantoin 50 MG twice a day for a UTI. On the 11th day I developed a red rash a... 5 replies
nitrofurantoin mono mcr 100 for staph infection related UTIs
My doctor told me that Cipro or Bactim are very good for treating UTIs caused by E-Coli, but that Nitrofurantoin Mono_MC... 4 replies
My doctor told me that Cipro or Bactim are very good for treating UTIs caused by E-Coli, but that Nitrofurantoin Mono_MC... 4 replies
Nitrofurantoin-Vaginal itching?
Hello! I was prescribed Nitrofurantoin for a UTI and I’m on my third day of taking it. My UTI symptoms seem to be ... 4 replies
Hello! I was prescribed Nitrofurantoin for a UTI and I’m on my third day of taking it. My UTI symptoms seem to be ... 4 replies
Nitrofurantoin dosing
I read the prescription directions wrong. I'm taking nitrofurantoin for a UTI in pregnancy. The directions say every... 3 replies
I read the prescription directions wrong. I'm taking nitrofurantoin for a UTI in pregnancy. The directions say every... 3 replies
Nitrofurantoin mono mcr 100 mg for uti
Hello, I was previously prescribed ciprofloxacin, for a uti caused by ecoli. After this didn't work, went back to th... 3 replies
Hello, I was previously prescribed ciprofloxacin, for a uti caused by ecoli. After this didn't work, went back to th... 3 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.