0024-5812 : 24 Hr Aplenzin 522 mg Extended Release Tablet
| NDC: | 0024-5812 |
| Labeler: | Sanofi-aventis U.S. LLC |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Aplenzin Aplenzin |
| Dosage Form: | Oral Tablet, Extended Release |
| Application #: | NDA022108 |
| Rev. Date: |
Appearance:
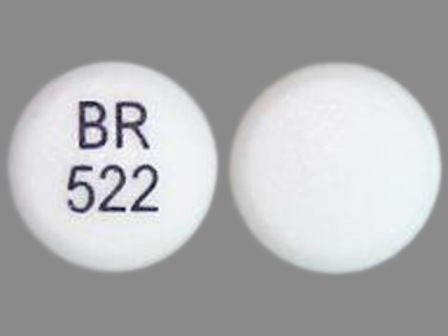
| Markings: | BR;522 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 11 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0024-5812-07: 7 TABLET, EXTENDED RELEASE IN 1 BOTTLE (0024‑5812‑07)
- 0024-5812-30: 30 TABLET, EXTENDED RELEASE IN 1 BOTTLE (0024‑5812‑30)
Active Ingredients:
- Bupropion Hydrobromide
Dosage Strength:
- 522 mg
Inactive Ingredients:
- Ethylcellulose (100 Mpa.s)
- Glyceryl Behenate
- Polyvinyl Alcohol
- Polyethylene Glycols
- Povidone
- Dibutyl Sebacate
Pharmaceutical Classes:
- Aminoketone [EPC]
- Dopamine Uptake Inhibitors [MoA]
- Increased Dopamine Activity [PE]
- Increased Norepinephrine Activity [PE]
- Norepinephrine Uptake Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 0024-5810 24 Hr Aplenzin 174 mg Extended Release Tablet by Sanofi-aventis U.S. LLC
- 0024-5811 24 Hr Aplenzin 348 mg Extended Release Tablet by Sanofi-aventis U.S. LLC
- 0187-5810 Aplenzin 174 mg Oral Tablet, Extended Release by Valeant Pharmaceuticals North America LLC
- 0187-5811 Aplenzin 348 mg Oral Tablet, Extended Release by Valeant Pharmaceuticals North America LLC
- 0187-5812 Aplenzin 522 mg Oral Tablet, Extended Release by Valeant Pharmaceuticals North America LLC
- 16590-922 24 Hr Aplenzin 348 mg Extended Release Tablet by Stat Rx USA LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0024-5811Next: 0024-5820 >
Related Discussions:
Is Aplenzin (Bupropion Hydrobromide) ER a narcotic?
My mother brought to my attention that anything with the word "hydro" in any medication could be a narcotic. I h... 3 replies
My mother brought to my attention that anything with the word "hydro" in any medication could be a narcotic. I h... 3 replies
Bupropion XL by Cipla Pharmaceuticals
My pharmacy has been giving me refills of the generic bupropion for my last two refills that are manufactured by CIPLA. ... 47 replies
My pharmacy has been giving me refills of the generic bupropion for my last two refills that are manufactured by CIPLA. ... 47 replies
Bupropion XL 300 by Lupin (L016)
Has anyone tried Bupropion XL 300 by Lupin - a round pill marked L016? Just received these from Walgreen's internet ... 33 replies
Has anyone tried Bupropion XL 300 by Lupin - a round pill marked L016? Just received these from Walgreen's internet ... 33 replies
Bupropion and decreased alcohol consumption
I've recently started bupropion, it's been a few weeks. I think I experienced every single side effect the drug ... 8 replies
I've recently started bupropion, it's been a few weeks. I think I experienced every single side effect the drug ... 8 replies
bupropion - actavis vs sandoz
I have been taking bupropuin 150 mg 2 times daily made by actavis for over 6 months and doing very well with it last wee... 7 replies
I have been taking bupropuin 150 mg 2 times daily made by actavis for over 6 months and doing very well with it last wee... 7 replies
Bupropion HCL SR 150 Mg purple/rnd/rph {b41} : SIDE EFFECTS
I have been to a neurologist, a rheumatologist, i've been for an M.R.I., and also been for more blood tests than I c... 7 replies
I have been to a neurologist, a rheumatologist, i've been for an M.R.I., and also been for more blood tests than I c... 7 replies
Bupropion Xl - change in generic tablet recently
Have been using Bupropian HCL XL 300 mg tabs for over 5 years. The markins have always been A102 from Anchen manufacturi... 7 replies
Have been using Bupropian HCL XL 300 mg tabs for over 5 years. The markins have always been A102 from Anchen manufacturi... 7 replies
Bupropion HCL XL 150 Mg Wpl 3331
Current Bupropion HCL XL150 MB TABGLO are yellow oblong pills that are press-stamped 681. New Rx refill is Bupropion HC ... 6 replies
Current Bupropion HCL XL150 MB TABGLO are yellow oblong pills that are press-stamped 681. New Rx refill is Bupropion HC ... 6 replies
bupropion identification
i have received bupropion sr 150 and the have no stamp on them, they are round and light yellow. how do I know if they a... 6 replies
i have received bupropion sr 150 and the have no stamp on them, they are round and light yellow. how do I know if they a... 6 replies
Bupropion helped me quit smoking
I started taking Bupropion a week ago and I quit smoking after 9 years. This works! ## Congrats James, great to hear a s... 5 replies
I started taking Bupropion a week ago and I quit smoking after 9 years. This works! ## Congrats James, great to hear a s... 5 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.