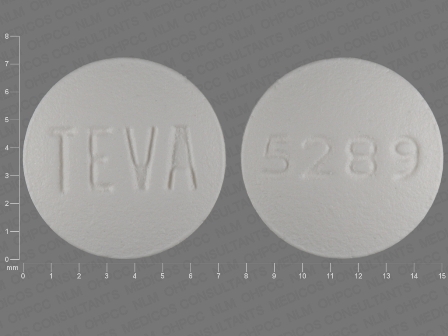
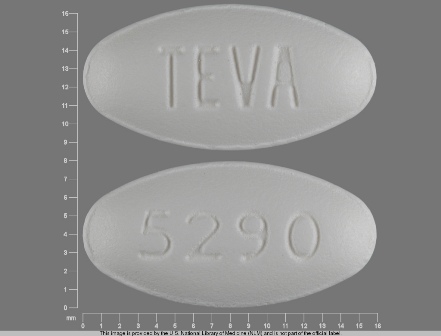
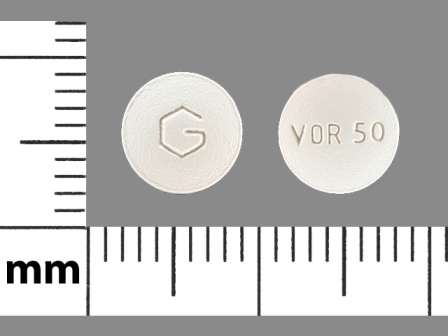
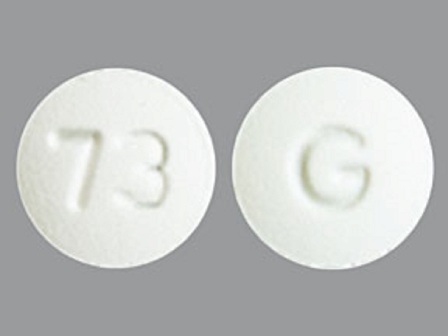
Voriconazole
FDA Approved: * April 22, 2010Pharm Company: * MATRIX LABS LTD
Category: Antifungal
Voriconazole, sold under the brand name Vfend among others, is an antifungal medication used to treat a number of fungal infections.[2] This includes aspergillosis, candidiasis, coccidioidomycosis, histoplasmosis, penicilliosis, and infections by Scedosporium or Fusarium.[2] It can be taken by mouth or used by injection into a vein.[2] Common side effects include vision problems, nausea, abdominal pain, rash, headache, and seeing or hearing thin... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.2 Discussions
Dosage List
Related Brands
Drugs with the same active ingredientsPopular Topics
Voriconazole and sun sensitivity 3 REPLIES
3 REPLIES
Does anyone have experience with skin sun sensitivity and extended voriconazole use for propholactic purposes (in immune...
Voriconazole use problems!
Just finished my first 4 months on Vori & prednisone. Other than what my docs tell me I have no other source to find...