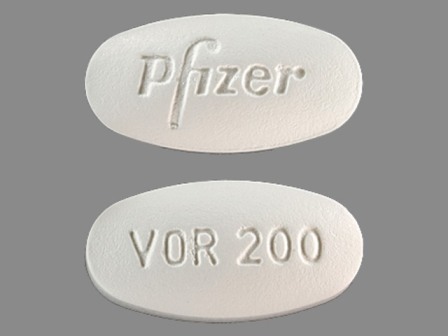
VFEND
Active Ingredient(s): VoriconazoleFDA Approved: * May 24, 2002
Pharm Company: * PFIZER
Category: Antifungal
Voriconazole, sold under the brand name Vfend among others, is an antifungal medication used to treat a number of fungal infections.[2] This includes aspergillosis, candidiasis, coccidioidomycosis, histoplasmosis, penicilliosis, and infections by Scedosporium or Fusarium.[2] It can be taken by mouth or used by injection into a vein.[2] Common side effects include vision problems, nausea, abdominal pain, rash, headache, and seeing or hearing thin... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion