Rapamune
Active Ingredient(s): SirolimusFDA Approved: * September 15, 1999
Pharm Company: * WYETH PHARMS INC
Category: Immunosuppressive
Sirolimus, also known as rapamycin and sold under the brand name Rapamune among others, is a macrolide compound that is used to coat coronary stents, prevent organ transplant rejection, treat a rare lung disease called lymphangioleiomyomatosis, and treat perivascular epithelioid cell tumor (PEComa).[1][2][8][9] It has immunosuppressant functions in humans and is especially useful in preventing the rejection of kidney transplants.... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List

Rapamune 0.5 mg Oral Tablet
NDC: 0008-1040
Labeler:
Wyeth Pharmaceuticals Company, a Subsidiary of Pfizer Inc.
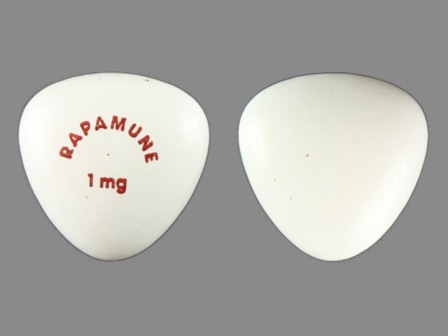
Rapamune 1 mg Oral Tablet
NDC: 0008-1041
Labeler:
Wyeth Pharmaceuticals Company, a Subsidiary of Pfizer Inc.

Rapamune 2 mg Oral Tablet
NDC: 0008-1042
Labeler:
Wyeth Pharmaceuticals Company, a Subsidiary of Pfizer Inc.

Rapamune 1 mg/ml Oral Solution
NDC: 0008-1030
Labeler:
Wyeth Pharmaceuticals Company, a Subsidiary of Pfizer Inc.