Mucinex Dm
Active Ingredient(s): Dextromethorphan Hydrobromide + GuaifenesinFDA Approved: * April 29, 2004
Pharm Company: * ADAMS LABS INC
Category: Allergies, Cough & Cold
Guaifenesin, sold under the brand name Mucinex, among others,[3] is a medication used as an expectorant, intended to help cough out phlegm from the airways.[3] Chemically it is an ether of guaiacol and glycerine. It is unclear if it decreases coughing.[3] Use is not recommended in children less than 6 years old.[4] It is often used in combination with other medications.[3] It is taken by mouth.[3] ... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.2 Discussions
Dosage List
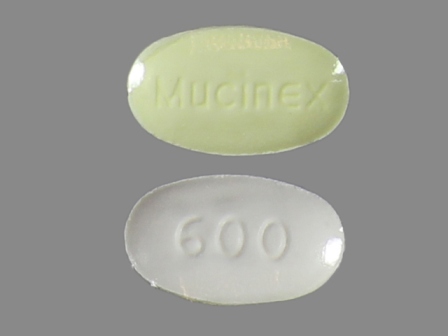









Related Brands
Drugs with the same active ingredients- Amibid DM
- Antituss DM
- Aquatab DM
- Benylin Expectorant
- Bidex DM
- Cheracol-D
- Diabetic Tussin DM
- DuraDex
- Duratuss DM
- ExeFen-DM
- Genatuss DM
- Guiatuss DM Oral Solution
- Guiatuss DM Oral Syrup
- Halotussin DM
- Muco-Fen DM
- Mytussin DM
- Naldecon Senior DX
- Phanatuss
- Respa-DM
- Rhinosyn-DMX
- Robitussin DM
- Safe Tussin 30
- Silexin
- Siltussin DM
- Tolu-Sed DM
- Tuss-DM
- Tussi-Organidin DM NR
- Tussin DM
- Tussin DM Clear
- Vicks 44E Pediatric
Popular Topics
Can MucinexDM and Lisinopril be taken together? ## Hello, Patsy! How are you? I didn't find any problems or interact...
I've been sick and im on 100 mg of Methadone a day and I was wondering if it would be o.k. to take with Mucinex-DM w...
You reciently changed you packageing of MucinexDM to come in a box that the pills have to be punched out of and not in t...
BLUE SIDE HAS #4 WHITE SIDE HAS #600 ## I would like to know if this medicine is just for cough or if it also breaks up ...
can I take some mucinex after I took levodopa ## I didn't find any interactions or problems listed between the two m...
I’ve been taking Azithromycin for 5 days, for bronchitis, but it’s doing nothing for my cough. I’m up ...
Can I take Mucinex along with Tylenol & Amoxicillin / Clavulanate Potassium Tablets, USP 875 mg/125 mg? ## Hi Nancy,...
Is there a harmful interaction between Mucinex D (Guaifenesin/Pseudoephedrine HCI) and HCTZ (hydrochlorothiazide). I tak...
Can I take these two drugs at the same time? ## Hello, Lizz! How are you? I didn't find any interactions listed betw...
Can I also take hydrochlorot with mucinex ## I didn't find any interactions listed between the two, but you may want...