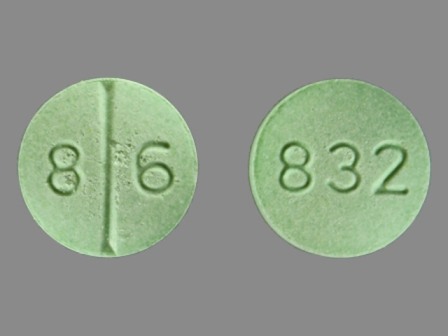
Fluoxymesterone
FDA Approved: * October 21, 1983Pharm Company: * USL PHARMA
Category: Men's Health
Fluoxymesterone, sold under the brand names Halotestin and Ultandren among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, breast cancer in women, and anemia.[1] It is taken by mouth.[1] Side effects of fluoxymesterone include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[1] It can al... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List