Entacapone
FDA Approved: * July 16, 2012Pharm Company: * SUN PHARMA GLOBAL
Category: Parkinsons
Entacapone, sold under the brand name Comtan among others, is a medication commonly used in combination with other medications for the treatment of Parkinson's disease.[1] Entacapone together with levodopa and carbidopa allows levodopa to have a longer effect in the brain and reduces Parkinson's disease signs and symptoms for a greater length of time than levodopa and carbidopa therapy alone.[1] Entacapone is a selective and reversible inhibitor of the enzyme c... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List
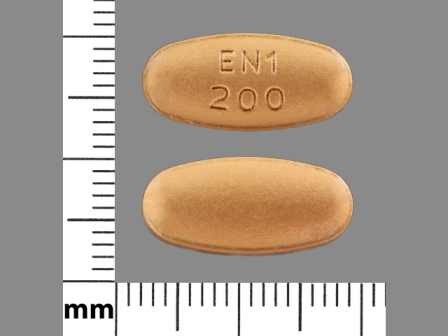


Entacapone 200 mg Oral Tablet, Film Coated
NDC: 43353-996
Labeler:
Aphena Pharma Solutions - Tennessee, LLC




Related Brands
Drugs with the same active ingredientsPopular Topics
carbidopa-levadopa-entacapone-
prescribed for Parkinson Disease...