Betaxolol
FDA Approved: * June 30, 2000Pharm Company: * AKORN
Category: Blood Pressure
Betaxolol is a selective beta1 receptor blocker used in the treatment of hypertension and glaucoma.[1] Being selective for beta1 receptors, it typically has fewer systemic side effects than non-selective beta-blockers, for example, not causing bronchospasm (mediated by beta2 receptors) as timolol may. Betaxolol also shows greater affinity for beta1 receptors than metoprolol. In addition to its effect on the heart, betaxolol reduces the pressure within the eye (intraocular pre... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List
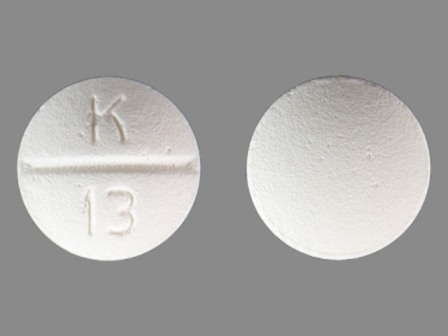
Betaxolol Hydrochloride 10 mg (Betaxolol 8.94 mg) Oral Tablet
NDC: 10702-013
Labeler:
Kvk-tech, Inc.

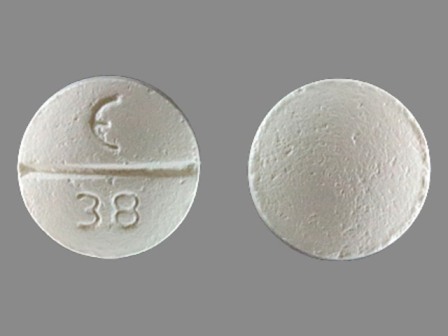
Betaxolol Hydrochloride 10 mg (Betaxolol 8.94 mg) Oral Tablet
NDC: 42806-038
Labeler:
Epic Pharma LLC

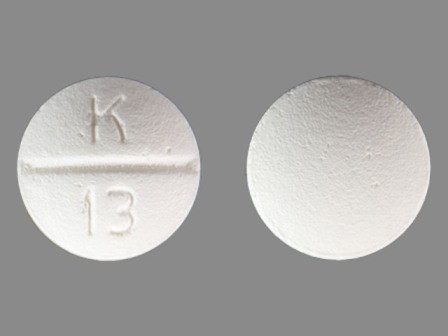
Betaxolol Hydrochloride 10 mg (Betaxolol 8.94 mg) Oral Tablet
NDC: 60429-753
Labeler:
Golden State Medical Supply, Inc.
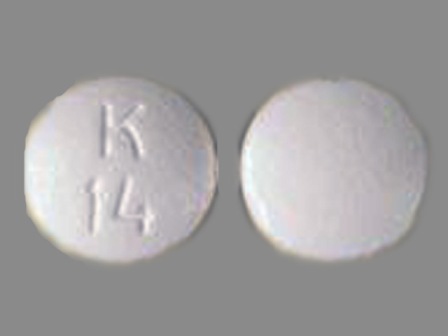
Betaxolol Hydrochloride (Betaxolol 17.88 mg) Oral Tablet
NDC: 60429-754
Labeler:
Golden State Medical Supply, Inc.



