70518-2124 : Tacrolimus 1 mg Oral Capsule
| NDC: | 70518-2124 |
| Labeler: | Remedyrepack Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Tacrolimus Tacrolimus |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA091195 |
| Rev. Date: |
Appearance:
| Markings: | TCR;1 |
| Shapes: |
Capsule |
| Colors: |
 White White |
| Size (mm): | 11 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
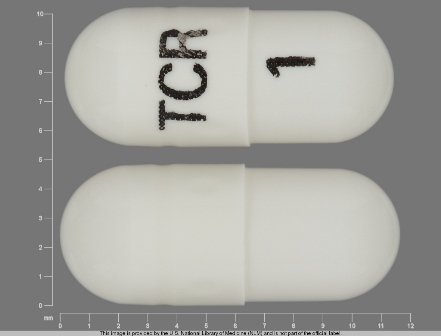
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 70518-2124-0: 30 CAPSULE IN 1 BLISTER PACK (70518‑2124‑0)
- 70518-2124-1: 100 POUCH IN 1 BOX (70518‑2124‑1) > 1 CAPSULE IN 1 POUCH (70518‑2124‑2)
Active Ingredients:
- Tacrolimus
Dosage Strength:
- 1 mg
Inactive Ingredients:
- Croscarmellose Sodium
- Hypromellose 2910 (5 Mpa.s)
- Magnesium Stearate
- Lactose Monohydrate
- Gelatin
- Titanium Dioxide
- Sodium Lauryl Sulfate /
Pharmaceutical Classes:
- Calcineurin Inhibitor Immunosuppressant [EPC]
- Calcineurin Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 70518-1168 Tacrolimus 1 mg Oral Capsule by Remedyrepack Inc.
- 70518-2593 Tacrolimus 1 mg Oral Capsule by Remedyrepack Inc.
- 70518-2996 Tacrolimus 1 mg Oral Capsule by Remedyrepack Inc.
- 70518-3022 Tacrolimus .5 mg Oral Capsule by Remedyrepack Inc.
- 70518-3216 Tacrolimus 5 mg Oral Capsule by Remedyrepack Inc.
- 70518-3388 Tacrolimus 1 mg Oral Capsule by Remedyrepack Inc.
- 0093-3428 Tacrolimus .3 mg/g Topical Ointment by Teva Pharmaceuticals USA, Inc.
- 0093-3429 Tacrolimus 1 mg/g Topical Ointment by Teva Pharmaceuticals USA, Inc.
- 0168-0416 Tacrolimus 1 mg/g Topical Ointment by E. Fougera & Co. a Division of Fougera Pharmaceuticals Inc.
- 0168-0417 Tacrolimus .3 mg/g Topical Ointment by E. Fougera & Co. a Division of Fougera Pharmaceuticals Inc.
- 0179-0079 Tacrolimus 1 mg (As Anhydrous Tacrolimus) Oral Capsule by Kaiser Foundation Hospitals
- 0378-2045 Tacrolimus 0.5 mg (As Anhydrous Tacrolimus) Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-2046 Tacrolimus 1 mg (As Anhydrous Tacrolimus) Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-2047 Tacrolimus 5 mg (As Anhydrous Tacrolimus) Oral Capsule by Mylan Pharmaceuticals Inc.
- 0591-3359 Tacrolimus 5 mg (As Anhydrous Tacrolimus) Oral Capsule by Watson Laboratories, Inc.
- 0781-2102 Tacrolimus 0.5 mg (As Anhydrous Tacrolimus) Oral Capsule by Sandoz Inc
- 0781-2103 Tacrolimus 1 mg (As Anhydrous Tacrolimus) Oral Capsule by Sandoz Inc
- 0781-2104 Tacrolimus 5 mg (As Anhydrous Tacrolimus) Oral Capsule by Sandoz Inc
- 0781-9302 Tacrolimus 0.5 mg (As Anhydrous Tacrolimus) Oral Capsule by Sandoz Inc
- 0781-9303 Tacrolimus 1 mg (As Anhydrous Tacrolimus) Oral Capsule by Sandoz Inc
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 70518-2123Next: 70518-2125 >
Related Discussions:
stopping tacrolimus
Are there any withdrawals or side effects from stopping Tacrolimus? I had a heart tx and I'm still on Rappamune, Cel... 1 reply
Are there any withdrawals or side effects from stopping Tacrolimus? I had a heart tx and I'm still on Rappamune, Cel... 1 reply
Pomegranate interactions with Tacrolimus and Mycophenolate
Can I take a tonic that includes pomegranate supplement when taking Tacrolimus and Mycophenolate anti-kidney-rejection m... 1 reply
Can I take a tonic that includes pomegranate supplement when taking Tacrolimus and Mycophenolate anti-kidney-rejection m... 1 reply
Forskolin, Atenolol, Amlodipine, Tacrolimus, Bactrim, Humalog & Cellcept
I had a kidney transplant and am on the following medications: Tacrolimus, cellcept, amlodipine, atenolol, bactrim &... 1 reply
I had a kidney transplant and am on the following medications: Tacrolimus, cellcept, amlodipine, atenolol, bactrim &... 1 reply
Tacrolimus Ointment 0 1% w/w
Tacrolimus Ointment 0 1% w/w is help full for depigmentation of skin..??...
Tacrolimus Ointment 0 1% w/w is help full for depigmentation of skin..??...
tacrolimus assy
please send the tacrolimus assay method for research work...
please send the tacrolimus assay method for research work...
Tacrolimus assay
Does anyone know how to remove the excipients from tacrolimus prior to running it on HPLC? I have found that susequent r...
Does anyone know how to remove the excipients from tacrolimus prior to running it on HPLC? I have found that susequent r...
tacrolimus
kidney transplant sapresant...
kidney transplant sapresant...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.