59762-1720 : Eplerenone 50 mg Oral Tablet
| NDC: | 59762-1720 |
| Labeler: | Greenstone LLC |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Eplerenone Eplerenone |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA021437 |
| Rev. Date: |
Appearance:
| Markings: | G;50mg |
| Shapes: |
Diamond (4 sides) |
| Colors: |
 Yellow Yellow |
| Size (mm): | 9 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
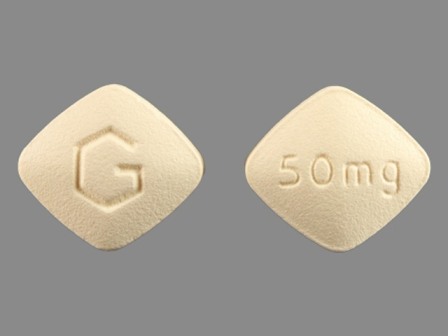
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 59762-1720-1: 30 TABLET, FILM COATED IN 1 BOTTLE (59762‑1720‑1)
- 59762-1720-2: 90 TABLET, FILM COATED IN 1 BOTTLE (59762‑1720‑2)
Active Ingredients:
- Eplerenone
Dosage Strength:
- 50 mg
Inactive Ingredients:
- Lactose
- Cellulose, Microcrystalline
- Croscarmellose Sodium
- Hypromelloses
- Sodium Lauryl Sulfate
- Talc
- Magnesium Stearate
- Titanium Dioxide
- Polyethylene Glycols
- Polysorbate 80
- Ferric Oxide Yellow
- Ferric Oxide Red
Pharmaceutical Classes:
- Aldosterone Antagonist [EPC]
- Aldosterone Antagonists [MoA]
Related Products:
Based on records with the same trade name.- 59762-1710 Eplerenone 25 mg Oral Tablet by Greenstone LLC
- 0185-5368 Eplerenone 25 mg Oral Tablet by Eon Labs, Inc.
- 0185-5369 Eplerenone 50 mg Oral Tablet by Eon Labs, Inc.
- 0378-1030 Eplerenone 25 mg Oral Tablet, Film Coated by Mylan Pharmaceuticals Inc.
- 0378-1031 Eplerenone 50 mg Oral Tablet, Film Coated by Mylan Pharmaceuticals Inc.
- 0832-0480 Eplerenone 25 mg Oral Tablet by Upsher-smith Laboratories, Inc.
- 0832-0481 Eplerenone 50 mg Oral Tablet by Upsher-smith Laboratories, Inc.
- 16729-293 Eplerenone 25 mg Oral Tablet, Film Coated by Accord Healthcare, Inc.
- 16729-294 Eplerenone 50 mg Oral Tablet, Film Coated by Accord Healthcare, Inc.
- 31722-049 Eplerenone 25 mg Oral Tablet, Film Coated by Camber Pharmaceuticals, Inc.
- 31722-050 Eplerenone 50 mg Oral Tablet, Film Coated by Camber Pharmaceuticals, Inc.
- 42254-011 Eplerenone 50 mg Oral Tablet by Rebel Distributors Corp.
- 42291-454 Eplerenone 25 mg Oral Tablet, Coated by Avkare
- 42291-455 Eplerenone 50 mg Oral Tablet, Coated by Avkare
- 51991-877 Eplerenone 25 mg Oral Tablet, Film Coated by Breckenridge Pharmaceutical, Inc.
- 51991-878 Eplerenone 50 mg Oral Tablet, Film Coated by Breckenridge Pharmaceutical, Inc.
- 54868-6065 Eplerenone 25 mg Oral Tablet by Physicians Total Care, Inc.
- 60505-2651 Eplerenone 25 mg Oral Tablet by Apotex Corp
- 60505-2652 Eplerenone 50 mg Oral Tablet by Apotex Corp
- 60687-451 Eplerenone 25 mg Oral Tablet, Film Coated by American Health Packaging
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 59762-1710Next: 59762-1735 >
Related Discussions:
inspra eplerenone
heart medication ## i have had a heart attack due to the wall of the artery collapseing and have had a stent now this is... 1 reply
heart medication ## i have had a heart attack due to the wall of the artery collapseing and have had a stent now this is... 1 reply
Alternative to Inspra (Eplerenone)
What would be an alternative to Inspra (Eplerenone) which is prescribed for treating high blood pressure and/or heart fa...
What would be an alternative to Inspra (Eplerenone) which is prescribed for treating high blood pressure and/or heart fa...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.