54569-6607 : Avodart .5 mg Oral Capsule, Liquid Filled
| NDC: | 54569-6607 |
| Labeler: | A-s Medication Solutions |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Avodart Avodart |
| Dosage Form: | Oral Capsule, Liquid Filled |
| Application #: | NDA021319 |
| Rev. Date: |
Appearance:
| Markings: | GX;CE2 |
| Shapes: |
Oval |
| Colors: |
 Yellow Yellow |
| Size (mm): | 19 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
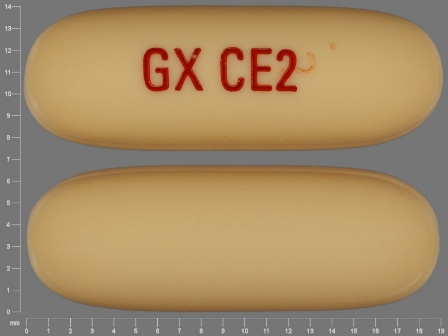
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 54569-6607-0: 30 CAPSULE, LIQUID FILLED IN 1 BOTTLE (54569‑6607‑0)
Active Ingredients:
- Dutasteride
Dosage Strength:
- .5 mg
Inactive Ingredients:
- Butylated Hydroxytoluene
- Ferric Oxide Yellow
- Gelatin
- Glycerin
- Caprylic/Capric Mono/Diglycerides
Pharmaceutical Classes:
- 5-alpha Reductase Inhibitor [EPC]
- 5-alpha Reductase Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 0173-0712 Avodart 0.5 mg Oral Capsule by Glaxosmithkline LLC
- 35356-210 Avodart 0.5 mg Oral Capsule by Lake Erie Medical & Surgcial Supply Dba Quality Care Products LLC
- 54868-5114 Avodart 0.5 mg Oral Capsule by Physicians Total Care, Inc.
- 68788-9044 Avodart 0.5 mg Oral Capsule by Preferred Pharmaceuticals, Inc
- 69189-0712 Avodart .5 mg Oral Capsule, Liquid Filled by Avera Mckennan Hospital
- 69784-712 Avodart .5 mg Oral Capsule, Liquid Filled by Woodward Pharma Services LLC
- 80725-712 Avodart .5 mg Oral Capsule, Liquid Filled by Waylis Therapeutics LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 54569-6604Next: 54569-6609 >
Related Discussions:
Dutas-T (Dutasteride and Tamsulosin)
I am 74 years old and I experienced difficulty urinating three years ago. For the last six months, I have been taking Du... 5 replies
I am 74 years old and I experienced difficulty urinating three years ago. For the last six months, I have been taking Du... 5 replies
temsunol d TAMSULOSIN HCL N DUTASTERIDE
I AM A PATIENt OF PROSTATE, DR RECOMMENDed ME URIMAX D BUT DISPENSARY PEOPLE GAVE ME TEMSUNOL D INSTEAD OS URIMAX D PLEA... 6 replies
I AM A PATIENt OF PROSTATE, DR RECOMMENDed ME URIMAX D BUT DISPENSARY PEOPLE GAVE ME TEMSUNOL D INSTEAD OS URIMAX D PLEA... 6 replies
Tamsulosin Hydrochloride 0.4 mg and Dutasteride 0.5 mg (URIMAX D)
I have problem of BPH, size is 28 gm weight and post void urine retention is 40 ml. I have been taking this combination ... 4 replies
I have problem of BPH, size is 28 gm weight and post void urine retention is 40 ml. I have been taking this combination ... 4 replies
Androgel and Avodart
I m 70 . I take Androgel 2.5 for low testosterone and libido . Now my urologist prescribed Avodart for some enlarged pro... 2 replies
I m 70 . I take Androgel 2.5 for low testosterone and libido . Now my urologist prescribed Avodart for some enlarged pro... 2 replies
side effects of concor 2.5 mg with avodart 0.5 mg and fuzata
Sir. What are the side effect of Concor cor 2.5 mg (beta 1 blocker) taken together with Avodart 0.5 mg and Fizatal XL 10...
Sir. What are the side effect of Concor cor 2.5 mg (beta 1 blocker) taken together with Avodart 0.5 mg and Fizatal XL 10...
dutasteride0 5mgwith metformin500mg
Metformin hydrochloride prolonged release 500mg & dutastride 0.5mg & tamsulosin hydrochloride prolonged release ...
Metformin hydrochloride prolonged release 500mg & dutastride 0.5mg & tamsulosin hydrochloride prolonged release ...
dutasteride tamsulosin
what does capsul look like?...
what does capsul look like?...
Avodart copyright
When will we be able to obtain Avodart as a generic? ## The patent for Avodart expires in 2013, so after that companies ... 1 reply
When will we be able to obtain Avodart as a generic? ## The patent for Avodart expires in 2013, so after that companies ... 1 reply
avodart
prostate medicine ## If you would like more information about this drug, please see the following pages: Summary Page: /... 1 reply
prostate medicine ## If you would like more information about this drug, please see the following pages: Summary Page: /... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.