50090-1644 : Amlodipine Besylate and Benazepril Hydrochloride Oral Capsule
| NDC: | 50090-1644 |
| Labeler: | A-s Medication Solutions |
| Product Type: | Human Prescription Drug |
| Drug Name: | Amlodipine Besylate and Benazepril Hydrochloride |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA090149 |
| Rev. Date: |
Appearance:
| Markings: | RDY;587 |
| Shapes: |
Capsule |
| Colors: |
 Brown Brown |
| Size (mm): | 19 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
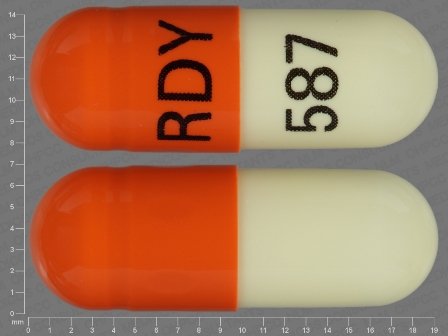
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 50090-1644-0: 90 CAPSULE IN 1 BOTTLE (50090‑1644‑0)
Active Ingredients:
- Amlodipine Besylate
- Benazepril Hydrochloride
Dosage Strength:
- 5 mg
- 40 mg
Inactive Ingredients:
- Silicon Dioxide
- Crospovidone
- Lactose Monohydrate
- Magnesium Stearate
- Cellulose, Microcrystalline
- Sodium Starch Glycolate Type a Potato
- Talc
- Fd&c Blue No. 1
- Fd&c Red No. 40
- Fd&c Yellow No. 6
- Gelatin
- Sodium Lauryl Sulfate
- Titanium Dioxide
Pharmaceutical Classes:
- Calcium Channel Antagonists [MoA]
- Dihydropyridine Calcium Channel Blocker [EPC]
- Dihydropyridines [Chemical/Ingredient]
- Angiotensin Converting Enzyme Inhibitor [EPC]
- Angiotensin-converting Enzyme Inhibitors [MoA]
- Decreased Blood Pressure [PE]
Related Products:
Based on records with the same trade name.- 50090-1038 Amlodipine Besylate and Benazepril Hydrochloride Oral Capsule by A-s Medication Solutions
- 50090-1041 Amlodipine Besylate and Benazepril Hydrochloride Oral Capsule by A-s Medication Solutions
- 50090-1463 Amlodipine Besylate and Benazepril Hydrochloride Oral Capsule by A-s Medication Solutions
- 50090-5137 Amlodipine Besylate and Benazepril Hydrochloride Oral Capsule by A-s Medication Solutions
- 50090-5357 Amlodipine Besylate and Benazepril Hydrochloride Oral Capsule by A-s Medication Solutions
- 50090-5389 Amlodipine Besylate and Benazepril Hydrochloride Oral Capsule by A-s Medication Solutions
- 50090-5404 Amlodipine Besylate and Benazepril Hydrochloride Oral Capsule by A-s Medication Solutions
- 50090-6185 Amlodipine Besylate and Benazepril Hydrochloride Oral Capsule by A-s Medication Solutions
- 50090-6427 Amlodipine Besylate and Benazepril Hydrochloride Oral Capsule by A-s Medication Solutions
- 0093-7370 Amlodipine (As Amlodipine Besylate) 2.5 mg / Benazepril Hydrochloride 10 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0093-7371 Amlodipine (As Amlodipine Besylate) 5 mg / Benazepril Hydrochloride 10 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0093-7372 Amlodipine (As Amlodipine Besylate) 5 mg / Benazepril Hydrochloride 20 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0093-7373 Amlodipine (As Amlodipine Besylate) 10 mg / Benazepril Hydrochloride 20 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0093-7670 Amlodipine (As Amlodipine Besylate) 5 mg / Benazepril Hydrochloride 40 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0093-7671 Amlodipine (As Amlodipine Besylate) 10 mg / Benazepril Hydrochloride 40 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0591-3757 Amlodipine (As Amlodipine Besylate) 2.5 mg / Benazepril Hydrochloride 10 mg Oral Capsule by Watson Laboratories, Inc.
- 0591-3758 Amlodipine (As Amlodipine Besylate) 5 mg / Benazepril Hydrochloride 10 mg Oral Capsule by Watson Laboratories, Inc.
- 0591-3759 Amlodipine (As Amlodipine Besylate) 5 mg / Benazepril Hydrochloride 20 mg Oral Capsule by Watson Laboratories, Inc.
- 0591-3760 Amlodipine (As Amlodipine Besylate) 10 mg / Benazepril Hydrochloride 20 mg Oral Capsule by Watson Laboratories, Inc.
- 0591-3761 Amlodipine (As Amlodipine Besylate) 5 mg / Benazepril Hydrochloride 40 mg Oral Capsule by Watson Laboratories, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 50090-1643Next: 50090-1645 >
Related Discussions:
amlodipine besylate and amlodipine/benazepril
Can amlodipine besylate and amlodipine/benazepril be taken at the same time? This is for my high blood pressure. ## Has ... 1 reply
Can amlodipine besylate and amlodipine/benazepril be taken at the same time? This is for my high blood pressure. ## Has ... 1 reply
is amlodipine besylate the same drug as benazepril
In USA my Dr. prescribed amlodipine besylate-benazepril 5-10mg now I live in Thailand and Dr. here has given me amlodipi... 1 reply
In USA my Dr. prescribed amlodipine besylate-benazepril 5-10mg now I live in Thailand and Dr. here has given me amlodipi... 1 reply
Is Amlodipine Besylate the same as Amlodipine Benazepril?
They are both a yellow and white pill used to be Orangish and white? ## Amlodipine Benazepril is actually 2 medications,... 8 replies
They are both a yellow and white pill used to be Orangish and white? ## Amlodipine Benazepril is actually 2 medications,... 8 replies
Amlodipine Benazepril vs Lisinopril
ARE THESE TWO DRUGS COMPATABLE? CAN I SWITCH FROM THE 1ST TO LISINOPRIL WITHOUT ANY COMPLICATIONS? ## Looking for a cost... 11 replies
ARE THESE TWO DRUGS COMPATABLE? CAN I SWITCH FROM THE 1ST TO LISINOPRIL WITHOUT ANY COMPLICATIONS? ## Looking for a cost... 11 replies
Amlodipine Benazepril Purple Capsule
purpleish colored 10/20 mg capsules ## perscribed by physician but do not have information ## Here is the link to the mo... 5 replies
purpleish colored 10/20 mg capsules ## perscribed by physician but do not have information ## Here is the link to the mo... 5 replies
Amlodipine Benazepril Treatment
How long does it usually take for your system to get used to the medication? ## Amlodipine and Benazapril are both medic... 2 replies
How long does it usually take for your system to get used to the medication? ## Amlodipine and Benazapril are both medic... 2 replies
Amlodipine/Benazepril by Dr. Reddy's Laboratories
My husband takes the blue generic version of amlodipine/benazepril. When the pharmacy filled it again they shipped Dr. R... 1 reply
My husband takes the blue generic version of amlodipine/benazepril. When the pharmacy filled it again they shipped Dr. R... 1 reply
amlodipine-benazepril vs amlodipine-benaz
Is there a difference between amlodipine-benaz and amlodipine-benazepril? ## Hello, Gordon! How are you? No, they are th... 1 reply
Is there a difference between amlodipine-benaz and amlodipine-benazepril? ## Hello, Gordon! How are you? No, they are th... 1 reply
Amlodipine Benazepril Safety
What are the side effects and safety precautions regarding Amlodipine Benazepril? ## Some side effects of the use of Aml... 1 reply
What are the side effects and safety precautions regarding Amlodipine Benazepril? ## Some side effects of the use of Aml... 1 reply
Amlodipine + Benazepril Uses and Side Effects
What is this drug I'm taking used for and what are the side effects? ## Amlodipine and Benazepril, a generic for Lot... 10 replies
What is this drug I'm taking used for and what are the side effects? ## Amlodipine and Benazepril, a generic for Lot... 10 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.
