0615-5591 : Primidone 50 mg Oral Tablet
| NDC: | 0615-5591 |
| Labeler: | Ncs Healthcare of Ky, Inc Dba Vangard Labs |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Primidone Primidone |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA084903 |
| Rev. Date: |
Appearance:
| Markings: | LAN;1301 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 6 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
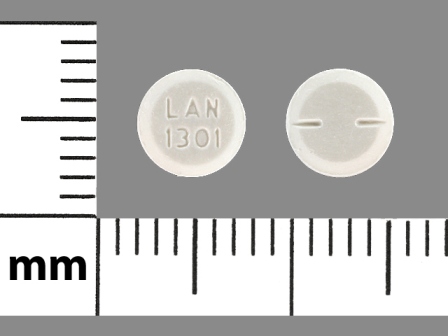
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0615-5591-30: 6 BLISTER PACK IN 1 BOX, UNIT‑DOSE (0615‑5591‑30) > 5 TABLET IN 1 BLISTER PACK
- 0615-5591-31: 31 TABLET IN 1 BLISTER PACK (0615‑5591‑31)
- 0615-5591-39: 30 TABLET IN 1 BLISTER PACK (0615‑5591‑39)
Active Ingredients:
- Primidone
Dosage Strength:
- 50 mg
Inactive Ingredients:
- Lactose Monohydrate
- Acacia
- Methylcellulose (400 Mpa.s)
- Magnesium Stearate
- Sodium Starch Glycolate Type a Potato
Pharmaceutical Classes:
- Anti-epileptic Agent [EPC]
- Decreased Central Nervous System Disorganized Electrical Activity [PE]
Related Products:
Based on records with the same trade name.- 0615-2521 Primidone 250 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0615-7738 Primidone 250 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0615-7936 Primidone 50 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0615-8206 Primidone 50 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0115-1030 Primidone 50 mg Oral Tablet by Global Pharmaceuticals
- 0115-1031 Primidone 250 mg Oral Tablet by Global Pharmaceuticals
- 0143-1482 Primidone 50 mg Oral Tablet by West-ward Pharmaceutical Corp
- 0143-1484 Primidone 250 mg Oral Tablet by West-ward Pharmaceutical Corp
- 0527-1231 Primidone 250 mg Oral Tablet by Lannett Company, Inc.
- 0527-1301 Primidone 50 mg Oral Tablet by Lannett Company, Inc.
- 0591-5321 Primidone 250 mg Oral Tablet by Watson Laboratories, Inc.
- 0603-5371 Primidone 50 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5372 Primidone 250 mg Oral Tablet by Qualitest Pharmaceuticals
- 0904-0560 Primidone 250 mg Oral Tablet by Major Pharmaceuticals
- 0904-5559 Primidone 50 mg Oral Tablet by Major Pharmaceuticals
- 0904-6207 Primidone 250 mg Oral Tablet by Major Pharmaceuticals
- 10135-522 Primidone 250 mg Oral Tablet by Marlex Pharmaceuticals Inc
- 10135-540 Primidone 50 mg Oral Tablet by Marlex Pharmaceuticals Inc
- 42291-508 Primidone 50 mg Oral Tablet by Avkare, Inc.
- 42291-509 Primidone 50 mg Oral Tablet by Avkare, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0615-5589Next: 0615-5592 >
Related Discussions:
Primidone 50mg tab
Tiny round white pill ## This is an anticonvulsant, here's the monograph link: ## I've been diagnosed with Essen... 3 replies
Tiny round white pill ## This is an anticonvulsant, here's the monograph link: ## I've been diagnosed with Essen... 3 replies
Primidone For mood?
I was on 1,500mg of primidone a few weeks ago and my doctor went down to 500mg.because of a med mistake. It was really h... 1 reply
I was on 1,500mg of primidone a few weeks ago and my doctor went down to 500mg.because of a med mistake. It was really h... 1 reply
primidone 250 mg side effects
List off side effects from talking primidone twice daily ## Hello, Malk! How are you? This is an anticonvulsant that may... 1 reply
List off side effects from talking primidone twice daily ## Hello, Malk! How are you? This is an anticonvulsant that may... 1 reply
primidone; 50mg; 5130
white; score and 5130 on 1 side; a or v on the other. ## Primidone Info Click Here... 1 reply
white; score and 5130 on 1 side; a or v on the other. ## Primidone Info Click Here... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.