0603-0235 : Bismuth Subsalicylate 262 mg Chewable Tablet
| NDC: | 0603-0235 |
| Labeler: | Qualitest Pharmaceuticals |
| Product Type: | Human OTC Drug |
| Drug Name: |  Pink Bismuth Pink Bismuth |
| Dosage Form: | Oral Tablet, Chewable |
| Application #: | part335 |
| Rev. Date: |
Appearance:
| Markings: | RH046 |
| Shapes: |
Round |
| Colors: |
 Pink Pink |
| Size (mm): | 15 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
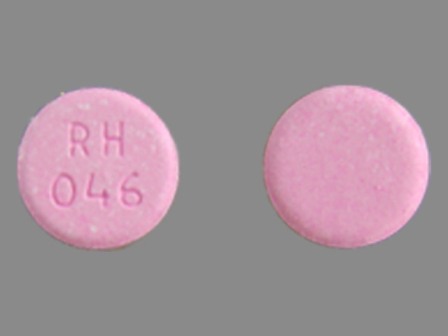
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0603-0235-16: 30 TABLET, CHEWABLE IN 1 CARTON (0603‑0235‑16)
Active Ingredients:
- Bismuth Subsalicylate
Dosage Strength:
- 262 mg
Inactive Ingredients:
- Aspartame
- Calcium Carbonate
- D&c Red No. 27
- Dextrates
- Magnesium Stearate
- Cellulose, Microcrystalline
- Silicon Dioxide
Related Products:
Based on records with the same trade name.- 0121-4803 Pink Bismuth 262 mg/15ml Oral Suspension by Pharmaceutical Associates, Inc.
- 0536-1810 Pink Bismuth 262 mg/15ml Oral Liquid by Rugby Laboratories, Inc.
- 53041-527 Pink-bismuth 262 mg/15ml Oral Liquid by Guardian Drug Company
- 57896-392 Pink Bismuth 262 mg/15ml Oral Liquid by Geri-care Pharmaceuticals, Corp
- 67510-0505 Pink Bismuth 262 mg/15ml Oral Liquid by Kareway Product, Inc.
- 68788-0190 Bismuth Subsalicylate 262 mg Chewable Tablet by Preferred Pharmaceuticals, Inc
- 70005-047 Pink Bismuth 262 mg/15ml Oral Liquid by We Care Distributor Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0603-0213Next: 0603-0241 >
Related Discussions:
composition of bismuth subsalicylate drug
This is a drug which is used for diarrhea,a antidiarrheal drug...
This is a drug which is used for diarrhea,a antidiarrheal drug...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.