0555-0483 : Amiloride Hydrochloride 5 mg / Hctz 50 mg Oral Tablet
| NDC: | 0555-0483 |
| Labeler: | Barr Laboratories Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: | Amiloride Hydrochloride and Hydrochlorothiazide |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA071111 |
| Rev. Date: |
Appearance:
| Markings: | 555;483;barr |
| Shapes: |
Round |
| Colors: |
 Yellow Yellow |
| Size (mm): | 9 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
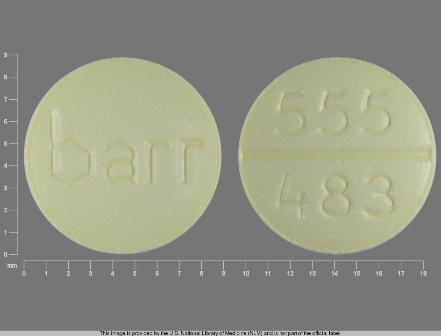
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0555-0483-02: 100 TABLET IN 1 BOTTLE (0555‑0483‑02)
- 0555-0483-05: 1000 TABLET IN 1 BOTTLE (0555‑0483‑05)
Active Ingredients:
- Amiloride Hydrochloride
- Hydrochlorothiazide
Dosage Strength:
- 5 mg
- 50 mg
Inactive Ingredients:
- Croscarmellose Sodium
- D&c Yellow No. 10
- Aluminum Oxide
- Lactose Monohydrate
- Magnesium Oxide
- Magnesium Stearate
- Cellulose, Microcrystalline
Pharmaceutical Classes:
- Decreased Renal K+ Excretion [PE]
- Increased Diuresis [PE]
- Potassium-sparing Diuretic [EPC]
- Thiazide Diuretic [EPC]
- Thiazides [CS]
Related Products:
Based on records with the same trade name.- 0378-0577 Amiloride Hydrochloride 5 mg / Hctz 50 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 50090-0513 Amiloride Hydrochloride and Hydrochlorothiazide Oral Tablet by A-s Medication Solutions
- 54569-3869 Amiloride Hydrochloride and Hydrochlorothiazide Oral Tablet by A-s Medication Solutions
- 54868-0667 Amiloride Hydrochloride 5 mg / Hctz 50 mg Oral Tablet by Physicians Total Care, Inc.
- 69189-0577 Amiloride Hydrochloride and Hydrochlorothiazide Oral Tablet by Avera Mckennan Hospital
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0555-0392Next: 0555-0484 >
Related Discussions:
Hydrochlorothiazide 12 5 Mg Side Effects
how do you take the med???and how late to take not to make you get up during the night???? ## When I was on them, my doc... 9 replies
how do you take the med???and how late to take not to make you get up during the night???? ## When I was on them, my doc... 9 replies
Hydrochlorothiazide/25mg effects?
What are the effects of this drug? Are there any side effects? ## half the 25 mg prescribed for HBP; need to know about ... 8 replies
What are the effects of this drug? Are there any side effects? ## half the 25 mg prescribed for HBP; need to know about ... 8 replies
Hydrochlorothiazide 25 Mg Side Effects
After after taking hydrochlorothiazide 25mg I HAVE RINGING In ears. Dry mouth, hearing loss. Not sure if this is related... 6 replies
After after taking hydrochlorothiazide 25mg I HAVE RINGING In ears. Dry mouth, hearing loss. Not sure if this is related... 6 replies
Hydrochlorothiazide And Triamterene By Sandoz Pharmaceuticals
My aunt has been taking the Sandoz Pharmaceutical, hydrochlorothiazide and triamterene for 13 yrs. The GG606, now the ph... 6 replies
My aunt has been taking the Sandoz Pharmaceutical, hydrochlorothiazide and triamterene for 13 yrs. The GG606, now the ph... 6 replies
HYDROCHLOROTHIAZIDE Missed Dose
If you do not take this medication for 3-5 days can it cause water retention right away? ## Yes,I agree,especially durin... 6 replies
If you do not take this medication for 3-5 days can it cause water retention right away? ## Yes,I agree,especially durin... 6 replies
Hydrochlorothiazide 25mg Uses
Is this a water pill or a high blood pressure pill? ## It actually lowers blood pressure by expelling water from the bod... 4 replies
Is this a water pill or a high blood pressure pill? ## It actually lowers blood pressure by expelling water from the bod... 4 replies
Hydrochlorothiazide 12.5mg Capsule
Need to Verify identification of Capsule: Small White Capsule with imprint Mylan 810 ## Pill Image You are correct, this... 4 replies
Need to Verify identification of Capsule: Small White Capsule with imprint Mylan 810 ## Pill Image You are correct, this... 4 replies
hydrochlorothiazide 12 5mg side effects
Prescribed hydrochlorothiazide 12.5mg for hypertension. Took for 1st 3 months with no problems outside of frequent urina... 3 replies
Prescribed hydrochlorothiazide 12.5mg for hypertension. Took for 1st 3 months with no problems outside of frequent urina... 3 replies
Hydrochlorothiazide Side Effects
I'm taking Hydrochlorothiazide for water retention due to a side effect from high estrogen levels in my birth contro... 3 replies
I'm taking Hydrochlorothiazide for water retention due to a side effect from high estrogen levels in my birth contro... 3 replies
Hydrochlorothiazide 12 5 Mg
Can dayquil and nitequil be used with this medacation? ## There are no interactions listed between using them, while on ... 3 replies
Can dayquil and nitequil be used with this medacation? ## There are no interactions listed between using them, while on ... 3 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.
