0378-0471 : Fenoprofen 600 mg Oral Tablet
| NDC: | 0378-0471 |
| Labeler: | Mylan Pharmaceuticals Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Fenoprofen Calcium Fenoprofen Calcium |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | ANDA072267 |
| Rev. Date: |
Appearance:
| Markings: | M471 |
| Shapes: |
Oval |
| Colors: |
 Orange Orange |
| Size (mm): | 20 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
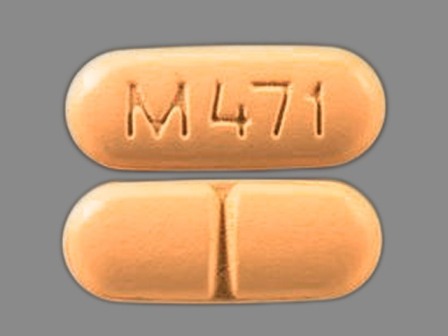
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0378-0471-01: 100 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (0378‑0471‑01)
Active Ingredients:
- Fenoprofen Calcium
Dosage Strength:
- 600 mg
Inactive Ingredients:
- Silicon Dioxide
- Croscarmellose Sodium
- Hypromellose
- Magnesium Stearate
- Cellulose, Microcrystalline
- Polyethylene Glycol
- Polysorbate 80
- Starch, Corn
- Sodium Lauryl Sulfate
- Titanium Dioxide
- Fd&c Yellow No. 6
Pharmaceutical Classes:
- Cyclooxygenase Inhibitors [MoA]
- Nonsteroidal Anti-inflammatory Compounds [Chemical/Ingredient]
- Nonsteroidal Anti-inflammatory Drug [EPC]
Related Products:
Based on records with the same trade name.- 0276-0501 Fenoprofen Calcium 200 mg Oral Capsule by Misemer Pharmaceuticals, Inc.
- 0276-0502 Fenoprofen Calcium 300 mg Oral Capsule by Misemer Pharmaceuticals, Inc.
- 0276-0503 Fenoprofen Calcium 400 mg Oral Capsule by Misemer Pharmaceuticals, Inc.
- 15014-400 Fenoprofen Calcium 400 mg Oral Capsule by Gentex Pharma
- 16571-688 Fenoprofen Calcium 400 mg Oral Capsule by Rising Pharma Holdings, Inc.
- 42195-100 Fenoprofen Calcium 400 mg Oral Capsule by Xspire Pharma LLC
- 42195-471 Fenoprofen Calcium 600 mg Oral Tablet, Film Coated by Xspire Pharma, LLC
- 50090-3572 Fenoprofen Calcium 400 mg Oral Capsule by A-s Medication Solutions
- 53217-169 Fenoprofen Calcium 400 mg Oral Capsule by Aidarex Pharmaceuticals LLC
- 54288-131 Fenoprofen Calcium 200 mg Oral Capsule by Bpi Labs LLC
- 54288-132 Fenoprofen Calcium 400 mg Oral Capsule by Bpi Labs LLC
- 55289-334 Fenoprofen 600 mg Oral Tablet by Pd-rx Pharmaceuticals, Inc.
- 63629-8016 Fenoprofen Calcium 400 mg Oral Capsule by Bryant Ranch Prepack
- 69101-400 Fenoprofen Calcium 400 mg Oral Capsule by Burke Therapeutics, LLC
- 69336-124 Fenoprofen Calcium 200 mg Oral Capsule by Sterling Knight Pharmaceuticals
- 71205-828 Fenoprofen Calcium 400 mg Oral Capsule by Proficient Rx Lp
- 71335-0307 Fenoprofen Calcium 400 mg Oral Capsule by Bryant Ranch Prepack
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0378-0467Next: 0378-0472 >
Related Discussions:
Calcium brand names available in India
I need to know all the calcium combination brand names that are available in india. ## Have you tried asking your doctor... 17 replies
I need to know all the calcium combination brand names that are available in india. ## Have you tried asking your doctor... 17 replies
CALCIUM CITRATE MALEATE PROPERTIES
JUST SEND THE DETAIL OF THE PROPERTIES OF CALCIUM CITRATE MALEATE WITH ITS PHARMACOLOGICAL VALUE AND ITS MODE OF ACTION ... 4 replies
JUST SEND THE DETAIL OF THE PROPERTIES OF CALCIUM CITRATE MALEATE WITH ITS PHARMACOLOGICAL VALUE AND ITS MODE OF ACTION ... 4 replies
Calcium deposit, calcium & magnesium gluconate, calcium hydroxycitrate
I was just shown an xray of my shoulder joint (the ball) that showed a calcium deposit creating excruciating pain. Tendi... 1 reply
I was just shown an xray of my shoulder joint (the ball) that showed a calcium deposit creating excruciating pain. Tendi... 1 reply
Calcium Cluconate Gel 2.5%
What is the prescription strength of calcium gluconate gel verses non-prescription strength. This is in reference to OSH... 1 reply
What is the prescription strength of calcium gluconate gel verses non-prescription strength. This is in reference to OSH... 1 reply
Calcium Carbonate 500 Mg
Hazards to your health if taking to many antacids with Calcium Carbonate 500 MG ## If you take too much, it may cause ov... 1 reply
Hazards to your health if taking to many antacids with Calcium Carbonate 500 MG ## If you take too much, it may cause ov... 1 reply
Calcium Acetate 667 Mg
Can capsules be opened and sprinkled in applesauce if you have trouble swallowing large capsules?Hoot ## In my opinion, ... 1 reply
Can capsules be opened and sprinkled in applesauce if you have trouble swallowing large capsules?Hoot ## In my opinion, ... 1 reply
Calcium Carbonate Vitamin D 3
I have been taking three D 500 CAL tablets per day for the last two years. This was prescribed to me for high PTH and lo... 1 reply
I have been taking three D 500 CAL tablets per day for the last two years. This was prescribed to me for high PTH and lo... 1 reply
calcium intake
I am 54 years old and i live in india.. i do not have any health problems. I had a pain in my left knee and that was dia... 1 reply
I am 54 years old and i live in india.. i do not have any health problems. I had a pain in my left knee and that was dia... 1 reply
calcium tabs
The best time to consume the calcium tabs.Is it in the day ( morning or noon or after or before the meals) or in the eve... 1 reply
The best time to consume the calcium tabs.Is it in the day ( morning or noon or after or before the meals) or in the eve... 1 reply
a calcium score of 286 serious
My mom that is 77, had a calcium scoring done and got a 286, can anyone explain what that means? ## Hello, Peg! How are ... 1 reply
My mom that is 77, had a calcium scoring done and got a 286, can anyone explain what that means? ## Hello, Peg! How are ... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.