61919-806 : Pantoprazole Sodium Dr 20 mg Oral Tablet, Delayed Release
| NDC: | 61919-806 |
| Labeler: | Direct Rx |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Pantoprazole Sodium Dr Pantoprazole Sodium Dr |
| Dosage Form: | Oral Tablet, Delayed Release |
| Application #: | ANDA090074 |
| Rev. Date: |
Appearance:
| Markings: | 96 |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 9 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
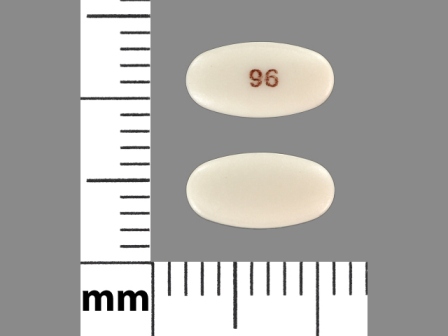
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 61919-806-30: 30 TABLET, DELAYED RELEASE IN 1 BOTTLE (61919‑806‑30)
- 61919-806-60: 60 TABLET, DELAYED RELEASE IN 1 BOTTLE (61919‑806‑60)
Active Ingredients:
- Pantoprazole Sodium
Dosage Strength:
- 20 mg
Inactive Ingredients:
- Calcium Stearate
- Hypromellose 2910 (3 Mpa.s)
- Sodium Carbonate
- Talc
- Crospovidone
- Hydroxypropyl Cellulose (1600000 Wamw)
- Mannitol
- Methacrylic Acid - Ethyl Acrylate Copolymer (1:1) Type a
- Propylene Glycol
- Titanium Dioxide
- Triethyl Citrate /
Pharmaceutical Classes:
- Proton Pump Inhibitor [EPC]
- Proton Pump Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 51655-501 Pantoprazole Sodium 40 mg Oral Tablet, Delayed Release by Northwind Pharmaceuticals, LLC
- 72189-514 Pantoprazole Sodium Dr Dr 40 mg Oral Tablet, Delayed Release by Direct_rx
- 80425-0085 Pantoprazole Sodium Dr 20 mg Oral Tablet, Delayed Release by Advanced Rx Pharmacy of Tennessee, LLC
- 80425-0133 Pantoprazole Sodium Dr 40 mg Oral Tablet, Delayed Release by Advanced Rx Pharmacy of Tennessee, LLC
- 80425-0162 Pantoprazole Sodium Dr 40 mg Oral Tablet, Delayed Release by Advanced Rx Pharmacy of Tennessee, LLC
- 80425-0179 Pantoprazole Sodium Dr 20 mg Oral Tablet, Delayed Release by Advanced Rx Pharmacy of Tennessee, LLC
- 80425-0278 Pantoprazole Sodium Dr 20 mg Oral Tablet, Delayed Release by Advanced Rx Pharmacy of Tennessee, LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 61919-805Next: 61919-807 >
Related Discussions:
Pantoprazole Sodium Domperidone Capsule
When is a good time to take pantoprazole sodium domperidone capsule (after dinner, lunch or before dinner)? ## Pantopraz... 16 replies
When is a good time to take pantoprazole sodium domperidone capsule (after dinner, lunch or before dinner)? ## Pantopraz... 16 replies
Pantoprazole Sodium Dr 40 Mg
does this product cause excessive sweating? ## Yes, it does list the possibility of causing increased sweating as a side... 1 reply
does this product cause excessive sweating? ## Yes, it does list the possibility of causing increased sweating as a side... 1 reply
pantocid tab(pantoprazole sodium)
uses of pantocid & side effects ## Pantoprazole is a proton pump inhibitor, it reduces the amount of acid that your ... 4 replies
uses of pantocid & side effects ## Pantoprazole is a proton pump inhibitor, it reduces the amount of acid that your ... 4 replies
pantoprazole tablets 40mg
i have stamac pain last two years, in 2009 i had taken endoscopy, at that doctor confirm :gr I Esophagitis, fatty liver,... 8 replies
i have stamac pain last two years, in 2009 i had taken endoscopy, at that doctor confirm :gr I Esophagitis, fatty liver,... 8 replies
Pantoprazole 40mg DR and EC
We were receiving the Pantoprazole 40mg EC tablets, yellow~oval~w434. The last refill we received the Pantoprazole 40mg ... 7 replies
We were receiving the Pantoprazole 40mg EC tablets, yellow~oval~w434. The last refill we received the Pantoprazole 40mg ... 7 replies
Pantoprazole Domperidone SR capsules
Each hard gelatin capsule contains Pantoprazole Sodium, Sesquihydrate equivalent to Pantoprazole 40mg. ## Pantoprazole i... 6 replies
Each hard gelatin capsule contains Pantoprazole Sodium, Sesquihydrate equivalent to Pantoprazole 40mg. ## Pantoprazole i... 6 replies
pantoprazole 40mg nor sure whether it is working
I have been prescribed pantoprazole 40mg to take one hour before my evening meal. The pill is small, yellow in colour wi... 6 replies
I have been prescribed pantoprazole 40mg to take one hour before my evening meal. The pill is small, yellow in colour wi... 6 replies
Pantoprazole 40mg Side Effect
Does this drug cause depression? ## Pantoprazole is a proton pump inhibitor that is used to reduce the amount of acid pr... 4 replies
Does this drug cause depression? ## Pantoprazole is a proton pump inhibitor that is used to reduce the amount of acid pr... 4 replies
pantoprazole 40mg pain on right side and back also
I have been taking pantoprazole 40mg for almost 3 months now and while it helped my abdominal pain after 1 month of use ... 3 replies
I have been taking pantoprazole 40mg for almost 3 months now and while it helped my abdominal pain after 1 month of use ... 3 replies
pantoprazole sod dr 40 mg and alcohol
Can I drink alcohol while on this medication? ## Hello, Sarah! How are you? If you have some condition caused by excess ... 3 replies
Can I drink alcohol while on this medication? ## Hello, Sarah! How are you? If you have some condition caused by excess ... 3 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.