60687-327 : Quetiapine Fumarate 25 mg Oral Tablet
| NDC: | 60687-327 |
| Labeler: | American Health Packaging |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Quetiapine Fumarate Quetiapine Fumarate |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA201109 |
| Rev. Date: |
Appearance:
| Markings: | LU;Y15 |
| Shapes: |
Round |
| Colors: |
 Pink Pink |
| Size (mm): | 6 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
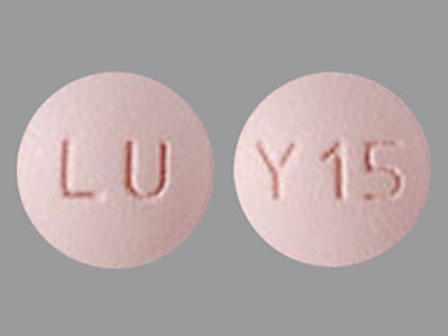
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 60687-327-01: 100 BLISTER PACK IN 1 BOX, UNIT‑DOSE (60687‑327‑01) > 1 TABLET IN 1 BLISTER PACK (60687‑327‑11)
Active Ingredients:
- Quetiapine Fumarate
Dosage Strength:
- 25 mg
Inactive Ingredients:
- Dibasic Calcium Phosphate Dihydrate
- Hypromellose 2910 (6 Mpa.s)
- Lactose Monohydrate
- Magnesium Stearate
- Microcrystalline Cellulose
- Polyethylene Glycol 400
- Povidone K30
- Sodium Starch Glycolate Type a Potato
- Titanium Dioxide
- Ferric Oxide Red
- Ferrosoferric Oxide
Pharmaceutical Classes:
- Atypical Antipsychotic [EPC]
Related Products:
Based on records with the same trade name.- 60687-338 Quetiapine Fumarate 50 mg Oral Tablet by American Health Packaging
- 60687-349 Quetiapine Fumarate 100 mg Oral Tablet by American Health Packaging
- 60687-360 Quetiapine Fumarate 200 mg Oral Tablet by American Health Packaging
- 60687-371 Quetiapine Fumarate 300 mg Oral Tablet by American Health Packaging
- 60687-382 Quetiapine Fumarate 400 mg Oral Tablet by American Health Packaging
- 60687-393 Quetiapine Fumarate 300 mg Oral Tablet by American Health Packaging
- 68084-530 Quetiapine (As Quetiapine Fumarate) 25 mg Oral Tablet by American Health Packaging
- 68084-531 Quetiapine (As Quetiapine Fumarate) 50 mg Oral Tablet by American Health Packaging
- 68084-532 Quetiapine (As Quetiapine Fumarate) 100 mg Oral Tablet by American Health Packaging
- 68084-533 Quetiapine (As Quetiapine Fumarate) 200 mg Oral Tablet by American Health Packaging
- 68084-534 Quetiapine (As Quetiapine Fumarate) 300 mg Oral Tablet by American Health Packaging
- 68084-535 Quetiapine (As Quetiapine Fumarate) 400 mg Oral Tablet by American Health Packaging
- 0054-0220 Quetiapine (As Quetiapine Fumarate) 25 mg Oral Tablet by Roxane Laboratories, Inc.
- 0054-0221 Quetiapine (As Quetiapine Fumarate) 100 mg Oral Tablet by Roxane Laboratories, Inc.
- 0054-0222 Quetiapine (As Quetiapine Fumarate) 200 mg Oral Tablet by Roxane Laboratories, Inc.
- 0054-0223 Quetiapine (As Quetiapine Fumarate) 300 mg Oral Tablet by Roxane Laboratories, Inc.
- 0054-0229 Quetiapine (As Quetiapine Fumarate) 50 mg Oral Tablet by Roxane Laboratories, Inc.
- 0054-0230 Quetiapine (As Quetiapine Fumarate) 400 mg Oral Tablet by Roxane Laboratories, Inc.
- 0093-2063 Quetiapine (As Quetiapine Fumarate) 25 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-8161 Quetiapine (As Quetiapine Fumarate) 25 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 60687-326Next: 60687-328 >
Related Discussions:
Quetiapine - Side Effects
My Doctor prescribed this med. for sleeplessness. About three weeks later I felt pressure in my head, felt faint, short ... 4 replies
My Doctor prescribed this med. for sleeplessness. About three weeks later I felt pressure in my head, felt faint, short ... 4 replies
Quetiapine shelf-life
I have recently been prescribed quetapine for sleeplessness-- 50 mg per night. It is functioning perfectly. I get 8 hour... 3 replies
I have recently been prescribed quetapine for sleeplessness-- 50 mg per night. It is functioning perfectly. I get 8 hour... 3 replies
quetiapine lupin
I have had issues with Lupin's bupropion XL, and now it seems I am having issues with Lupin's quetiapine. It see... 3 replies
I have had issues with Lupin's bupropion XL, and now it seems I am having issues with Lupin's quetiapine. It see... 3 replies
Quetiapine withdrawal? Don't even think about it!
I was prescribed 25mg Quetiapine, because I was still going down-hill with depression, and it seemed to do the trick. Ho... 3 replies
I was prescribed 25mg Quetiapine, because I was still going down-hill with depression, and it seemed to do the trick. Ho... 3 replies
Quetiapine fumarate 25 mg acc
What does the acc on quetiapine fumarate 25 mg acc mean? And is there a difference from the same thing w/ out the acc? #... 2 replies
What does the acc on quetiapine fumarate 25 mg acc mean? And is there a difference from the same thing w/ out the acc? #... 2 replies
quetiapine fumarate accord
I have been taking the generic for Seroquel 400mg, Quetiapine Fumarate, for over a year. It treats my depression, anxiet... 2 replies
I have been taking the generic for Seroquel 400mg, Quetiapine Fumarate, for over a year. It treats my depression, anxiet... 2 replies
quetiapine/ feeling drunk
Just started 100 mg I felt like I was drunk I don't like this feeling will it go away ## Hello, Crashette! How are y... 1 reply
Just started 100 mg I felt like I was drunk I don't like this feeling will it go away ## Hello, Crashette! How are y... 1 reply
Quetiapine (sleepless nights)
Hi there my dad has just been prescribed these tablets and has been in them for a few days now and keeps having sleeples... 1 reply
Hi there my dad has just been prescribed these tablets and has been in them for a few days now and keeps having sleeples... 1 reply
seroquel quetiapine
hi, I've been taking quetiapine now for a number of years. I started out taking the extended release version of the ... 2 replies
hi, I've been taking quetiapine now for a number of years. I started out taking the extended release version of the ... 2 replies
Seroquel/Quetiapine Brand
Hey. I take quetiapine (seroquel) for anxiety at 50 mg. I'm just wondering because I recently went to a different ph... 1 reply
Hey. I take quetiapine (seroquel) for anxiety at 50 mg. I'm just wondering because I recently went to a different ph... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.