55154-3516 : Avelox 400 mg Oral Tablet
| NDC: | 55154-3516 |
| Labeler: | Cardinal Health |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Avelox Avelox |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA021085 |
| Rev. Date: |
Appearance:
| Markings: | BAYER;M400 |
| Shapes: |
Oval |
| Colors: |
 Red Red |
| Size (mm): | 17 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
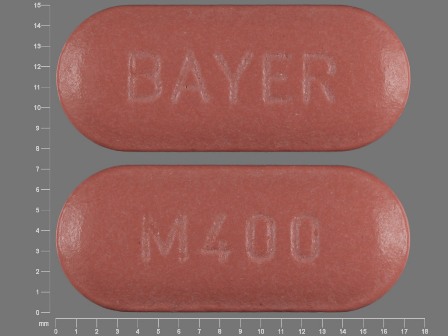
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 55154-3516-0: 10 BLISTER PACK IN 1 BAG (55154‑3516‑0) > 1 TABLET, FILM COATED IN 1 BLISTER PACK
Active Ingredients:
- Moxifloxacin Hydrochloride
Dosage Strength:
- 400 mg
Inactive Ingredients:
- Cellulose, Microcrystalline
- Lactose Monohydrate
- Croscarmellose Sodium
- Magnesium Stearate
- Hypromelloses
- Titanium Dioxide
- Polyethylene Glycols
- Ferric Oxide Red
Pharmaceutical Classes:
- Quinolone Antimicrobial [EPC]
- Quinolones [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 0085-1733 Avelox Abc Pack by Schering Plough Corporation
- 0085-1737 Avelox 400 mg/250ml Intravenous Injection, Solution by Schering Plough Corporation
- 49999-455 Avelox 400 mg Oral Tablet by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
- 50419-530 Avelox 400 mg Oral Tablet, Film Coated by Bayer Healthcare Pharmaceuticals Inc.
- 50419-537 Avelox 400 mg/250ml Intravenous Injection, Solution by Bayer Healthcare Pharmaceuticals Inc.
- 52125-525 Avelox 400 mg Oral Tablet by Remedyrepack Inc.
- 54868-4367 Avelox 400 mg Oral Tablet by Physicians Total Care, Inc.
- 55289-077 Avelox 400 mg Oral Tablet by Pd-rx Pharmaceuticals, Inc.
- 67296-0154 Avelox 400 mg Oral Tablet by Redpharm Drug Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 55154-3496Next: 55154-3525 >
Related Discussions:
avelox moxifloxacin hci
i want to know what this drug is and what is for ## Avelox is a fluoroquinolone antibiotic for treating bacterial infect... 4 replies
i want to know what this drug is and what is for ## Avelox is a fluoroquinolone antibiotic for treating bacterial infect... 4 replies
MOXIFLOXACIN HCI TABLETS
WHAT IS IT USED FOR ## can i take avelox moxifloxacin hci tablets for ear and throat infection. ## Moxifloxacin is an an... 2 replies
WHAT IS IT USED FOR ## can i take avelox moxifloxacin hci tablets for ear and throat infection. ## Moxifloxacin is an an... 2 replies
Moxifloxacin and effect on the uvula
I have no evidence of a causal link, but have a clear temporal link from the antibiotic to a degradation of the uvula co... 1 reply
I have no evidence of a causal link, but have a clear temporal link from the antibiotic to a degradation of the uvula co... 1 reply
Moxifloxacin wrong prescription
I recently had a sore throat, cold and fever. I found an old pack of my husband's Moxifloxacin 400 mg and on the fir... 1 reply
I recently had a sore throat, cold and fever. I found an old pack of my husband's Moxifloxacin 400 mg and on the fir... 1 reply
effect of moxifloxacin on pregnancy
i want to know about moxifloxacin and its side effects during pregnancy ## Moxifloxacin, also called Avelox, is in a cla... 1 reply
i want to know about moxifloxacin and its side effects during pregnancy ## Moxifloxacin, also called Avelox, is in a cla... 1 reply
switching from 5 days moxifloxacin to amoxicillin
Took moxifloxacin 5 days bad side effects is it safe too start amoxicillin as been 2 days since i took moxifloxacin. Had... 1 reply
Took moxifloxacin 5 days bad side effects is it safe too start amoxicillin as been 2 days since i took moxifloxacin. Had... 1 reply
Avelox 400 mg
moxifloxacin HCI ## i have an arrytmia should i take avelox ## Hi Maria, Avelox is a drug that can lead to many problems... 2 replies
moxifloxacin HCI ## i have an arrytmia should i take avelox ## Hi Maria, Avelox is a drug that can lead to many problems... 2 replies
Avelox and suntanning
I am taking Avelox for broncitis. I am scheduled to go away on vacation 4 days after finishing my treatment, am I able t... 1 reply
I am taking Avelox for broncitis. I am scheduled to go away on vacation 4 days after finishing my treatment, am I able t... 1 reply
avelox and champix
Has anyone ever experienced a problem mixing avelox and champex together ## Hi Paul, There is no known interactions betw... 1 reply
Has anyone ever experienced a problem mixing avelox and champex together ## Hi Paul, There is no known interactions betw... 1 reply
is avelox and acid reducer safe to take together
is avelox 400mg and acid reducer safe to take together ## Both Avelox and Acid Reducers can reduce magnesium levels in t... 1 reply
is avelox 400mg and acid reducer safe to take together ## Both Avelox and Acid Reducers can reduce magnesium levels in t... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.