0378-3288 : Etidronate Disodium 400 mg Oral Tablet
| NDC: | 0378-3288 |
| Labeler: | Mylan Pharmaceuticals Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: | Etidronate Disodium |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA075800 |
| Rev. Date: |
Appearance:
| Markings: | ED;400;G |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 16 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
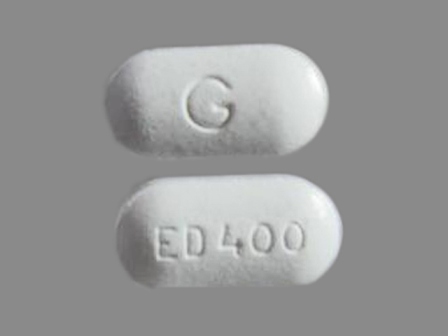
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0378-3288-91: 60 TABLET IN 1 BOTTLE, PLASTIC (0378‑3288‑91)
Active Ingredients:
- Etidronate Disodium
Dosage Strength:
- 400 mg
Inactive Ingredients:
- Magnesium Stearate
- Cellulose, Microcrystalline
- Starch, Corn
Pharmaceutical Classes:
- Bisphosphonate [EPC]
- Diphosphonates [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 0378-3286 Etidronate Disodium 200 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 55567-094 Etidronate Disodium 200 mg Oral Tablet by Genpharm Inc.
- 55567-095 Etidronate Disodium 400 mg Oral Tablet by Genpharm Inc.
- 68151-1521 Etidronate Disodium 200 mg Oral Tablet by Carilion Materials Management
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0378-3286Next: 0378-3340 >
Related Discussions:
etidronate side effects
Is the side effects the cause of my joint pain muscle and bone pain ## Hello, Brenda! How are you? Yes, it could be, the... 1 reply
Is the side effects the cause of my joint pain muscle and bone pain ## Hello, Brenda! How are you? Yes, it could be, the... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.
