Phenytek
Active Ingredient(s): PhenytoinFDA Approved: * December 28, 1998
Pharm Company: * MYLAN
Category: Anticonvulsant
Phenytoin (PHT), sold under the brand name Dilantin among others,[1] is an anti-seizure medication.[2] It is useful for the prevention of tonic-clonic seizures (also known as grand mal seizures) and focal seizures, but not absence seizures.[2] The intravenous form, fosphenytoin, is used for status epilepticus that does not improve with benzodiazepines.[2] It may also be used for certain heart arrhythmias or neuropathic pain.&... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.2 Discussions
Dosage List
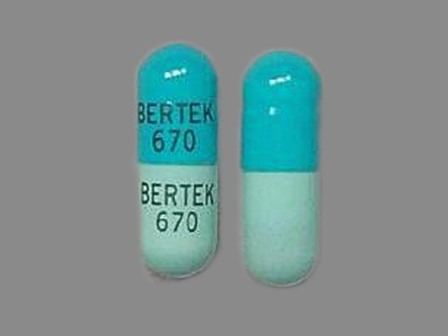
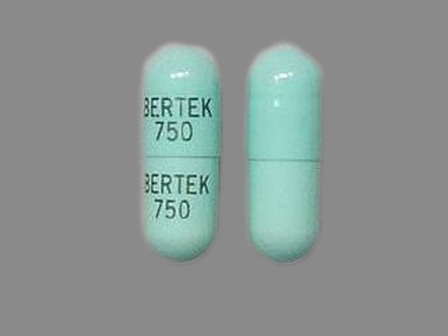

Phenytek 300 mg Oral Capsule, Extended Release
NDC: 58118-3750
Labeler:
Clinical Solutions Wholesale