Lyrica - NDC Database (Page 2)
112 records found
Start a Discussion
21695-662 Jun 10, 2005
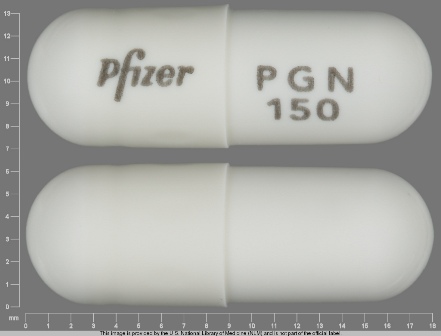
Lyrica 150 mg Oral Capsule by Rebel Distributors Corp
21695-663 Jun 10, 2005
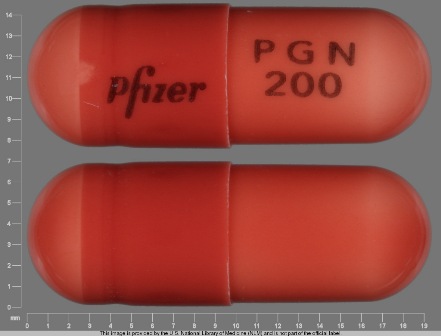
Lyrica 200 mg Oral Capsule by Rebel Distributors Corp
21695-664 Dec 30, 2004
Lyrica 300 mg Oral Capsule by Rebel Distributors Corp
24236-783 Aug 09, 2016
Lyrica 50 mg Oral Capsule by Remedyrepack Inc.
35356-053 Mar 19, 2012
Lyrica 25 mg Oral Capsule by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
35356-054 Feb 06, 2012
Lyrica 150 mg Oral Capsule by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
42254-134 Dec 30, 2004
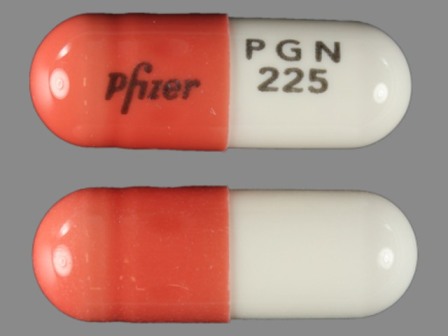
Lyrica 225 mg Oral Capsule by Rebel Distributors Corp
42549-612 Dec 30, 2004
Lyrica 75 mg Oral Capsule by Stat Rx USA LLC
42549-660 Dec 30, 2004
Lyrica 50 mg Oral Capsule by Stat Rx USA LLC
49999-895 Feb 10, 2011
Lyrica 75 mg Oral Capsule by Lake Erie Medical Dba Quality Care Products LLC
49999-905 Feb 06, 2012
Lyrica 100 mg Oral Capsule by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
49999-906 Sep 27, 2010
Lyrica 200 mg Oral Capsule by Lake Erie Medical Dba Quality Care Products LLC
49999-949 Nov 08, 2011
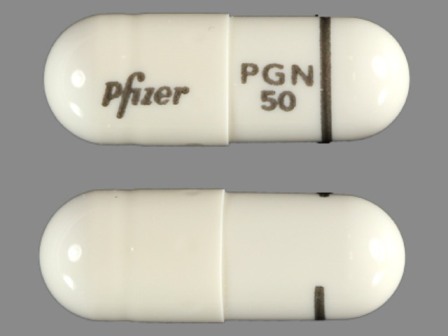
Lyrica 50 mg Oral Capsule by Lake Erie Medical & Surgical Supply, Dba Quality Care Products, LLC
50090-0993 Dec 30, 2004
Lyrica 75 mg Oral Capsule by A-s Medication Solutions LLC
50090-1229 Dec 30, 2004
Lyrica 50 mg Oral Capsule by A-s Medication Solutions LLC
50090-1230 Dec 30, 2004
Lyrica 50 mg Oral Capsule by A-s Medication Solutions LLC
50090-1231 Dec 30, 2004
Lyrica 100 mg Oral Capsule by A-s Medication Solutions LLC
50090-1232 Dec 30, 2004
Lyrica 100 mg Oral Capsule by A-s Medication Solutions LLC
50090-1304 Dec 30, 2004
Lyrica 150 mg Oral Capsule by A-s Medication Solutions LLC
50090-1305 Dec 30, 2004
Lyrica 150 mg Oral Capsule by A-s Medication Solutions LLC
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .