70518-1758 : Depakote 250 mg Oral Tablet, Extended Release
| NDC: | 70518-1758 |
| Labeler: | Remedyrepack Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Depakote ER Depakote ER |
| Dosage Form: | Oral Tablet, Extended Release |
| Application #: | NDA021168 |
| Rev. Date: |
Appearance:
| Markings: | a;HF |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 17 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
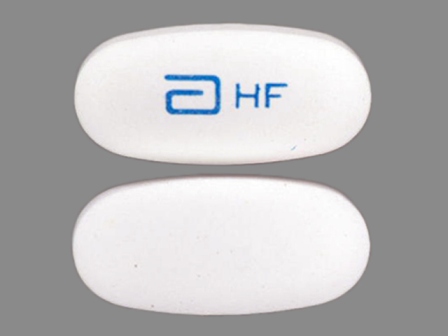
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 70518-1758-0: 30 TABLET, EXTENDED RELEASE IN 1 BLISTER PACK (70518‑1758‑0)
Active Ingredients:
- Divalproex Sodium
Dosage Strength:
- 250 mg
Inactive Ingredients:
- Titanium Dioxide
- Silicon Dioxide
- Potassium Sorbate
- Propylene Glycol
- Fd&c Blue No. 1
- Cellulose, Microcrystalline
- Triacetin
- Lactose, Unspecified Form
- Hypromellose, Unspecified
- Polyethylene Glycol, Unspecified /
Pharmaceutical Classes:
- Anti-epileptic Agent [EPC]
- Decreased Central Nervous System Disorganized Electrical Activity [PE]
- Mood Stabilizer [EPC]
Related Products:
Based on records with the same trade name.- 70518-1759 Depakote 500 mg Oral Tablet, Extended Release by Remedyrepack Inc.
- 70518-3754 Depakote 250 mg Oral Tablet, Extended Release by Remedyrepack Inc.
- 24236-411 Depakote 250 mg Oral Tablet, Extended Release by Remedyrepack Inc.
- 24236-979 Depakote 500 mg Oral Tablet, Extended Release by Remedyrepack Inc.
- 0074-7401 Depakote 250 mg Oral Tablet, Extended Release by Abbvie Inc.
- 0074-7402 Depakote 500 mg Oral Tablet, Extended Release by Abbvie Inc.
- 43353-310 Depakote 500 mg Oral Tablet, Extended Release by Aphena Pharma Solutions - Tennessee, LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 70518-1757Next: 70518-1759 >
Related Discussions:
Divalproex side effects Depakote generic
I was taking Depakote and topamax together. My liver quit processing this combo and my ammonia level in my blood shot up... 1 reply
I was taking Depakote and topamax together. My liver quit processing this combo and my ammonia level in my blood shot up... 1 reply
Divalproex Sodium Er 250mg
I had an Rx filled and was supposedly 250 mg divalproex sodium ER. It is orange, oval shape, and number 512 one side, bl... 1 reply
I had an Rx filled and was supposedly 250 mg divalproex sodium ER. It is orange, oval shape, and number 512 one side, bl... 1 reply
Divalproex Sodium Er 500 Mg
Divalproex delyed release 50mg, oval wite pill with zao6 onone sd, whatis this used for xacty ## Divalproex (Depakote) d... 1 reply
Divalproex delyed release 50mg, oval wite pill with zao6 onone sd, whatis this used for xacty ## Divalproex (Depakote) d... 1 reply
hydrocodone and divalproex sodium safe to tske compazine
I'm currently taking 500mg hydrocodone/apap every six hours for pain plus 250mg divalproex sodium twice daily. Is it... 1 reply
I'm currently taking 500mg hydrocodone/apap every six hours for pain plus 250mg divalproex sodium twice daily. Is it... 1 reply
Divalproex Er 500 Mg
divalproex Er 500mg tab myl ## divalproex er 500mg myl if i take 2mg xanax, soma, and hydroco/acetamin 10-325 how will i... 2 replies
divalproex Er 500mg tab myl ## divalproex er 500mg myl if i take 2mg xanax, soma, and hydroco/acetamin 10-325 how will i... 2 replies
divalproex er 500 mg vs pivalproex SOD ER 500 MG
My grandson has been taking divalproex 500 MG ER for bipolar disorder. We moved recently so I had to change drugstores. ... 1 reply
My grandson has been taking divalproex 500 MG ER for bipolar disorder. We moved recently so I had to change drugstores. ... 1 reply
Divalproex Er verses Divalproex regular
My husband has schitzo-affective disorder. The Depakote has helped him to remain somewhat stable with less manic episode... 1 reply
My husband has schitzo-affective disorder. The Depakote has helped him to remain somewhat stable with less manic episode... 1 reply
Divalproex Interactions
hello, I am on dextroamphetimine 40 mg a day for ADD symptoms, Tramadol PRN for back pain, Armour Thyroid 150 mcg for hy... 1 reply
hello, I am on dextroamphetimine 40 mg a day for ADD symptoms, Tramadol PRN for back pain, Armour Thyroid 150 mcg for hy... 1 reply
divalproex sod er 500 mg tab
I used Teva and it worked for me, in my next px they gave me the manufacturer Mylan and it doesn't work for me. I we... 2 replies
I used Teva and it worked for me, in my next px they gave me the manufacturer Mylan and it doesn't work for me. I we... 2 replies
Divalproex Sod Er Side Effects
my 17 year old son starrted ignoring going to school regularly. then shool counsellor told us to take him to any pshcatr... 2 replies
my 17 year old son starrted ignoring going to school regularly. then shool counsellor told us to take him to any pshcatr... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.