68682-409 : Chlordiazepoxide Hydrochloride 5 mg / Clidinium Bromide 2.5 mg Oral Capsule
| NDC: | 68682-409 |
| Labeler: | Oceanside Pharmaceuticals |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Chlordiazepoxide Hydrochloride and Clidinium Bromide Chlordiazepoxide Hydrochloride and Clidinium Bromide |
| Dosage Form: | Oral Capsule |
| Application #: | NDA012750 |
| Rev. Date: |
Appearance:
| Markings: | OPI;409 |
| Shapes: |
Capsule |
| Colors: |
 Green Green |
| Size (mm): | 14 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
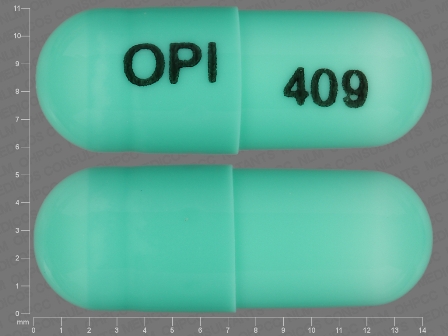
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 68682-409-10: 100 CAPSULE IN 1 BOTTLE, PLASTIC (68682‑409‑10)
Active Ingredients:
- Chlordiazepoxide Hydrochloride
- Clidinium Bromide
Dosage Strength:
- 5 mg
- 2.5 mg
Inactive Ingredients:
- Starch, Corn
- Lactose
- Talc
- Methylparaben
- Propylparaben
- Potassium Sorbate
- D&c Yellow No. 10
- Fd&c Blue No. 1
- Fd&c Green No. 3
Pharmaceutical Classes:
- Benzodiazepine [EPC]
- Benzodiazepines [CS]
- Anticholinergic [EPC]
- Decreased Parasympathetic Acetylcholine Activity [PE]
- GI Motility Alteration [PE]
- Digestive/GI System Activity Alteration [PE]
- Cholinergic Antagonists [MoA]
Related Products:
Based on records with the same trade name.- 10135-622 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Marlex Pharmaceuticals Inc
- 11534-197 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Sunrise Pharmaceutical, Inc.
- 23155-808 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Heritage Pharmaceuticals Inc. D/B/A Avet Pharmaceuticals Inc.
- 35573-325 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Burel Pharmaceuticals, Inc
- 42192-336 Chlordiazepoxide Hydrochloride 5 mg / Clidinium Bromide 2.5 mg Oral Capsule by Acella Pharmaceuticals, LLC
- 42494-409 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Cameron Pharmaceuticals, LLC
- 42582-300 Chlordiazepoxide Hydrochloride 5 mg / Clidinium Bromide 2.5 mg Oral Capsule by Bi-coastal Pharmaceutical Corporation
- 42582-301 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Bi-coastal Pharma International Limited Liability Company
- 43598-706 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Dr. Reddy's Laboratories Inc.
- 44183-401 Chlordiazepoxide Hydrochloride 5 mg / Clidinium Bromide 2.5 mg Oral Capsule by Macoven Pharmaceuticals
- 46708-744 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Alembic Pharmaceuticals Limited
- 50090-1528 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by A-s Medication Solutions
- 50090-6310 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by A-s Medication Solutions
- 51293-607 Chlordiazepoxide Hydrochloride 5 mg / Clidinium Bromide 2.5 mg Oral Capsule by Eci Pharmaceuticals LLC
- 54569-0430 Chlordiazepoxide Hydrochloride 5 mg / Clidinium Bromide 2.5 mg Oral Capsule by A-s Medication Solutions LLC
- 59651-524 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Aurobindo Pharma Limited
- 60219-1677 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Amneal Pharmaceuticals Ny LLC
- 60429-189 Chlordiazepoxide Hydrochloride 5 mg / Clidinium Bromide 2.5 mg Oral Capsule by Golden State Medical Supply, Inc.
- 60687-639 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by American Health Packaging
- 62135-696 Chlordiazepoxide Hydrochloride and Clidinium Bromide Oral Capsule by Chartwell Rx, LLC
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 68682-392Next: 68682-421 >
Related Discussions:
Chlordiazepoxide-clidinium substitute
Mr doctor wrote prescription for Librax and did not specify brand name only. My local pharmacy dispensed Chlordiazepoxid... 8 replies
Mr doctor wrote prescription for Librax and did not specify brand name only. My local pharmacy dispensed Chlordiazepoxid... 8 replies
Chlordiazepoxide Clidinium Caps Side Effects
chlordiazepoxide clidinium caps can these affect your blood pressure ## Some adverse reactions reported during therapy w... 3 replies
chlordiazepoxide clidinium caps can these affect your blood pressure ## Some adverse reactions reported during therapy w... 3 replies
Chlordiazepoxide Clidinium Capsule RE369
I have always filled this prescription and gotten small green capsules. Today I received yellow capsules with RE369 on t... 3 replies
I have always filled this prescription and gotten small green capsules. Today I received yellow capsules with RE369 on t... 3 replies
Chlordiazepoxide/Clidinium caps
Yes, it's ridiculous that both medicare and united health care will not pay for this! Or the generic librax. I have ... 1 reply
Yes, it's ridiculous that both medicare and united health care will not pay for this! Or the generic librax. I have ... 1 reply
Chlordiazepoxide-Clidinium 5 mg-2.5 mg
Does this medication have any interactions with the following drugs: Lamictal, Lithium Carbonate, Glucophage, Buspirone?... 1 reply
Does this medication have any interactions with the following drugs: Lamictal, Lithium Carbonate, Glucophage, Buspirone?... 1 reply
Chlordiazepoxide + Clidinium Information
It is Generic for Librax®, (Chlordiazepoxide HCl + Clidinium HBr). It's Strength is 5mg/2.5mg. It is a combinat... 5 replies
It is Generic for Librax®, (Chlordiazepoxide HCl + Clidinium HBr). It's Strength is 5mg/2.5mg. It is a combinat... 5 replies
Chlordiazepoxide HCl and Clidinium Bromide Capsules
Green cap A-018 ## Just to comfirm, the pill in description is Chlordiazepoxide + Clidinium (5 mg - 2.5 mg). To view inf... 5 replies
Green cap A-018 ## Just to comfirm, the pill in description is Chlordiazepoxide + Clidinium (5 mg - 2.5 mg). To view inf... 5 replies
Generic For Librax-chlordiazepoxide clidinium
I am trying to get a prescription program. I am on medicare. None of the prescrption programs cover the drug listed abov... 1 reply
I am trying to get a prescription program. I am on medicare. None of the prescrption programs cover the drug listed abov... 1 reply
Chlordiazepoxide affecting libido
Took 25mg tabs of librium for a week. Noticed that my libido disappeared. So i stopped taking it. How long will it take ... 1 reply
Took 25mg tabs of librium for a week. Noticed that my libido disappeared. So i stopped taking it. How long will it take ... 1 reply
Chlordiazepoxide yellow capsule
re389 ## Yes, these are made by a pharmaceutical company called Rivers Edge, this the RE 369 on the capsule. Chlordiazep... 1 reply
re389 ## Yes, these are made by a pharmaceutical company called Rivers Edge, this the RE 369 on the capsule. Chlordiazep... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.