66993-070 : Mefenamic Acid 250 mg Oral Capsule
| NDC: | 66993-070 |
| Labeler: | Prasco Laboratories |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Mefenamic Acid Mefenamic Acid |
| Dosage Form: | Oral Capsule |
| Application #: | NDA015034 |
| Rev. Date: |
Appearance:
| Markings: | FHPC400;PONSTEL |
| Shapes: |
Capsule |
| Colors: |
 White White |
| Size (mm): | 17 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
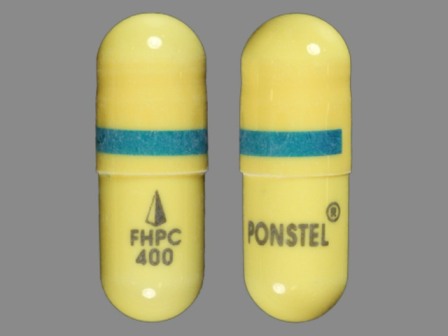
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 66993-070-30: 30 CAPSULE IN 1 BOTTLE (66993‑070‑30)
Active Ingredients:
- Mefenamic Acid
Dosage Strength:
- 250 mg
Inactive Ingredients:
- Lactose
- Citric Acid Monohydrate
- D&c Yellow No. 10
- Fd&c Blue No. 1
- Fd&c Red No. 3
- Fd&c Yellow No. 6
- Gelatin
- Glyceryl Monooleate
- Silicon Dioxide
- Sodium Benzoate
- Sodium Lauryl Sulfate
- Titanium Dioxide
Pharmaceutical Classes:
- Cyclooxygenase Inhibitors [MoA]
- Nonsteroidal Anti-inflammatory Compounds [Chemical/Ingredient]
- Nonsteroidal Anti-inflammatory Drug [EPC]
Related Products:
Based on records with the same trade name.- 0276-0509 Mefenamic Acid 250 mg Oral Capsule by Misemer Pharmaceuticals, Inc
- 0574-0195 Mefenamic Acid 250 mg Oral Capsule by Paddock Laboratories, LLC.
- 0603-4372 Mefenamic Acid 250 mg Oral Capsule by Qualitest Pharmaceuticals
- 0677-1934 Mefenamic Acid 250 mg Oral Capsule by Sciele Pharma Inc
- 42571-258 Mefenamic Acid 250 mg Oral Capsule by Micro Labs Limited
- 51991-839 Mefenamic Acid 250 mg Oral Capsule by Breckenridge Pharmaceutical, Inc.
- 60258-890 Mefenamic Acid 250 mg Oral Capsule by Cypress Pharmaceutical, Inc.
- 62250-677 Mefenamic Acid 250 mg Oral Capsule by Belcher Pharmaceuticals, LLC
- 64380-790 Mefenamic Acid 250 mg Oral Capsule by Strides Pharma Science Limited
- 68180-185 Mefenamic Acid 250 mg Oral Capsule by Lupin Pharmaceuticals Inc
- 69499-317 Mefenamic Acid 250 mg Oral Capsule by Solubiomix
- 80425-0306 Mefenamic Acid 250 mg Oral Capsule by Advanced Rx Pharmacy of Tennessee, LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 66993-068Next: 66993-075 >
Related Discussions:
mefenamic acid ponstan sf
mechanism of action ## Ponstan SF is a product manufactured in the Philippines, that is classified under Nonsteroidal An... 1 reply
mechanism of action ## Ponstan SF is a product manufactured in the Philippines, that is classified under Nonsteroidal An... 1 reply
R.M.Mefenamic Acid Shelf Life
want to know Raw Material Expiry / shelf life for Mefenamic Acid as per drug act ## Can I get expiry period of Raw Mater... 1 reply
want to know Raw Material Expiry / shelf life for Mefenamic Acid as per drug act ## Can I get expiry period of Raw Mater... 1 reply
Adco Mefenamic Acid 250mg during pregnancy
I'm 4 weeks pregnant and I was experiencing some bleeding and pain and cramping as if I was getting my period. I too... 4 replies
I'm 4 weeks pregnant and I was experiencing some bleeding and pain and cramping as if I was getting my period. I too... 4 replies
Adco-Mefenamic Acid 250mg and periods
I went to the Dr 2 months back to ask about my period pains and my skin problem and he said my skin will be fine and pre... 1 reply
I went to the Dr 2 months back to ask about my period pains and my skin problem and he said my skin will be fine and pre... 1 reply
pontalon mefenamic acid 500mg
I have been prescribed pontolon mefenamic acid 500mg for elbow bursitis. Is this a this a good cure ## I hurt my front t... 1 reply
I have been prescribed pontolon mefenamic acid 500mg for elbow bursitis. Is this a this a good cure ## I hurt my front t... 1 reply
Pontalon Mefenamic Acid
i have very very bad tooth pain. can i take mefanamic 250mg with pontalon?????. ## Mefenamic Acid is the active ingredie... 1 reply
i have very very bad tooth pain. can i take mefanamic 250mg with pontalon?????. ## Mefenamic Acid is the active ingredie... 1 reply
Pontalon Mefenamic Acid 250mg
I got a bad toothaches and was prescript Pontalon Mefenamic Acid 250mg, the next day i went for tooth extraction and was... 1 reply
I got a bad toothaches and was prescript Pontalon Mefenamic Acid 250mg, the next day i went for tooth extraction and was... 1 reply
1 tablet of mefenamic acid can harm a pregnant women?
Hi! I'm 2 week delayed my last menstrual period is oct.28 November to december 14 im missed my period. I think I am ... 2 replies
Hi! I'm 2 week delayed my last menstrual period is oct.28 November to december 14 im missed my period. I think I am ... 2 replies
Paracetamol And Mefenamic Acid
ive experienced fainting, diarrhea, severe abdominal cramps on the first day of my period starting again. Earlier i foun... 6 replies
ive experienced fainting, diarrhea, severe abdominal cramps on the first day of my period starting again. Earlier i foun... 6 replies
pontalon 500mg. Mefenamic acid
I'm having a bad tooth achne. Can I take pontalon 500mg as currently I'm taking aspirin tablet to thin down my b... 2 replies
I'm having a bad tooth achne. Can I take pontalon 500mg as currently I'm taking aspirin tablet to thin down my b... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.