64764-171 : Kapidex 30 mg Enteric Coated Capsule
| NDC: | 64764-171 |
| Labeler: | Takeda Pharmaceuticals America, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Dexilant Dexilant |
| Dosage Form: | Oral Capsule, Delayed Release |
| Application #: | NDA022287 |
| Rev. Date: |
Appearance:
| Markings: | TAP;30 |
| Shapes: |
Capsule |
| Colors: |
 Blue / Blue /
 Gray Gray |
| Size (mm): | 16 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
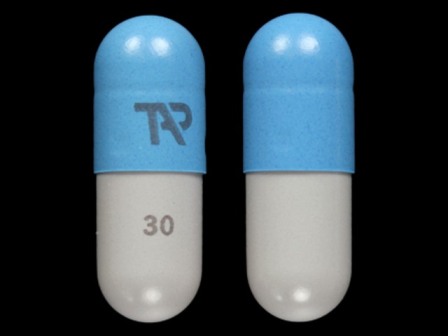
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 64764-171-00: 7 CAPSULE, DELAYED RELEASE IN 1 BOTTLE (64764‑171‑00)
- 64764-171-01: 5 BLISTER PACK IN 1 TRAY (64764‑171‑01) > 4 CAPSULE, DELAYED RELEASE IN 1 BLISTER PACK
- 64764-171-03: 1 BOTTLE IN 1 CARTON (64764‑171‑03) > 5 CAPSULE, DELAYED RELEASE IN 1 BOTTLE
- 64764-171-11: 10 BLISTER PACK IN 1 CARTON (64764‑171‑11) > 10 CAPSULE, DELAYED RELEASE IN 1 BLISTER PACK
- 64764-171-19: 1000 CAPSULE, DELAYED RELEASE IN 1 BOTTLE (64764‑171‑19)
- 64764-171-30: 30 CAPSULE, DELAYED RELEASE IN 1 BOTTLE (64764‑171‑30)
- 64764-171-90: 90 CAPSULE, DELAYED RELEASE IN 1 BOTTLE (64764‑171‑90)
Active Ingredients:
- Dexlansoprazole
Dosage Strength:
- 30 mg
Inactive Ingredients:
- Methacrylic Acid - Methyl Methacrylate Copolymer (1:1)
- Methacrylic Acid - Methyl Methacrylate Copolymer (1:2)
- Methacrylic Acid - Ethyl Acrylate Copolymer (1:1) Type a
- Magnesium Carbonate
- Sucrose
- Hydroxypropyl Cellulose, Low Substituted
- Titanium Dioxide
- Hydroxypropyl Cellulose (Type H)
- Hypromellose 2910 (6 Mpa.s)
- Talc
- Polyethylene Glycol 8000
- Triethyl Citrate
- Polysorbate 80
- Silicon Dioxide
- Carrageenan
- Potassium Chloride
- Ferric Oxide Red
- Aluminum Oxide
- Fd&c Blue No. 2
Pharmaceutical Classes:
- Proton Pump Inhibitor [EPC]
- Proton Pump Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 64764-175 Kapidex 60 mg Enteric Coated Capsule by Takeda Pharmaceuticals America, Inc.
- 50090-4374 Dexilant 60 mg Oral Capsule, Delayed Release by A-s Medication Solutions
- 54868-6152 Kapidex 60 mg Enteric Coated Capsule by Physicians Total Care, Inc.
- 55154-5154 Dexilant 60 mg Oral Capsule, Delayed Release by Cardinal Health
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 64764-158Next: 64764-175 >
Related Discussions:
deilxant (dexlansoprazole)
What is deilxant dr 60 mg capsule used for? ## Hello, Ernestine! How are you? The FDA classifies this medication as a pr... 1 reply
What is deilxant dr 60 mg capsule used for? ## Hello, Ernestine! How are you? The FDA classifies this medication as a pr... 1 reply
Dexilant 60mg
I am looking for an over the counter alternative for Dexilant 60mg cap. Is there one available? ## Hello, Quadcities1! H... 4 replies
I am looking for an over the counter alternative for Dexilant 60mg cap. Is there one available? ## Hello, Quadcities1! H... 4 replies
dexilant causing more pain
Ive been diagnosed with erosive gastritis. Before the diagnosis I was on prilosec 20 mg for 7 months. The acid went away... 3 replies
Ive been diagnosed with erosive gastritis. Before the diagnosis I was on prilosec 20 mg for 7 months. The acid went away... 3 replies
Dexilant 30mg vs Omeprazole 40mg
Is Omeprazole stronger than Dexilant 30mg? If I switch from Omeprazole 40mg to Dexilant 30mg will I have rebound acid? I... 2 replies
Is Omeprazole stronger than Dexilant 30mg? If I switch from Omeprazole 40mg to Dexilant 30mg will I have rebound acid? I... 2 replies
dexilant 60mg and omeprazole 20mg
I was diagnosed with EE (esophogial erosion) the doctor put me on dexilant (60 mg) and i am still experiencing a problem... 2 replies
I was diagnosed with EE (esophogial erosion) the doctor put me on dexilant (60 mg) and i am still experiencing a problem... 2 replies
Dexilant and Apple Cider Vinegar
I take Dexilant for GERD (hiatus hernia and acid reflux). Is there a risk in also taking Apple Cider Vinegar while on De... 1 reply
I take Dexilant for GERD (hiatus hernia and acid reflux). Is there a risk in also taking Apple Cider Vinegar while on De... 1 reply
dexilant concerns
I was given a weeks sample and then a prescription for 60mg. The Dr. made a mistake on the prescription and put down 2xd... 1 reply
I was given a weeks sample and then a prescription for 60mg. The Dr. made a mistake on the prescription and put down 2xd... 1 reply
Dexilant with Welchol and no gallbladder
Is it safe to take Dexilant and Welchol together without a gallbladder? Any suggestions or information about possible si... 1 reply
Is it safe to take Dexilant and Welchol together without a gallbladder? Any suggestions or information about possible si... 1 reply
dexilant 60mg out dated dexilant
l cannot afford the price of 60mg dexilant - have samples dated 2011 . stored in dark dry place. my condition is severe ... 1 reply
l cannot afford the price of 60mg dexilant - have samples dated 2011 . stored in dark dry place. my condition is severe ... 1 reply
dexilant 60 mg
Can I take 2 of the 60mg of dexilant in one day ## 60mgs is the maximum recommended daily dose. Are you still having aci... 1 reply
Can I take 2 of the 60mg of dexilant in one day ## 60mgs is the maximum recommended daily dose. Are you still having aci... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.