63629-7896 : Cartia 120 mg Oral Capsule, Extended Release
| NDC: | 63629-7896 |
| Labeler: | Bryant Ranch Prepack |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Cartia Xt Cartia Xt |
| Dosage Form: | Oral Capsule, Extended Release |
| Application #: | ANDA074752 |
| Rev. Date: |
Appearance:
| Markings: | Andrx;597;120;mg |
| Shapes: |
Capsule |
| Colors: |
 White / White /
 Orange Orange |
| Size (mm): | 18 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
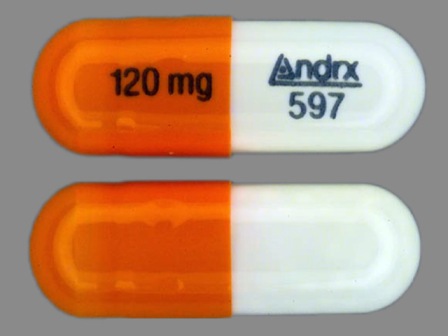
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 63629-7896-1: 30 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE (63629‑7896‑1)
- 63629-7896-2: 90 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE (63629‑7896‑2)
Active Ingredients:
- Diltiazem Hydrochloride
Dosage Strength:
- 120 mg
Inactive Ingredients:
- Acetyltributyl Citrate
- Ammonio Methacrylate Copolymer Type B
- D&c Red No. 28
- D&c Yellow No. 10
- Ethylcellulose (10 Mpa.s)
- Fd&c Blue No. 1
- Fd&c Blue No. 2
- Fd&c Red No. 40
- Gelatin, Unspecified
- Magnesium Stearate
- Methacrylic Acid - Methyl Methacrylate Copolymer (1:2)
- Propylene Glycol
- Polysorbate 80
- Starch, Corn
- Sucrose
- Talc
- Titanium Dioxide /
Pharmaceutical Classes:
- Calcium Channel Antagonists [MoA]
- Calcium Channel Blocker [EPC]
Related Products:
Based on records with the same trade name.- 71335-0879 Cartia 180 mg Oral Capsule, Extended Release by Bryant Ranch Prepack
- 50090-3051 Cartia 120 mg Oral Capsule, Extended Release by A-s Medication Solutions
- 50090-3124 Cartia 180 mg Oral Capsule, Extended Release by A-s Medication Solutions
- 61786-915 Cartia 120 mg Oral Capsule, Extended Release by Remedyrepack Inc.
- 70518-1537 Cartia 120 mg Oral Capsule, Extended Release by Remedyrepack Inc.
- 72189-344 Cartia Xt 300 mg Oral Capsule, Extended Release by Directrx
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 63629-7895Next: 63629-7902 >
Related Discussions:
diltiazem er/xr and cartia xt
Is there a difference between these 2 meds I was on diliazem 120 er/xr for 4 years and had no problems and now the med w... 2 replies
Is there a difference between these 2 meds I was on diliazem 120 er/xr for 4 years and had no problems and now the med w... 2 replies
Diltiazem / Cartia / Cardizem
I was taking Diltiazem 120 24 HR. I just filled my prescription at Safeway and they gave me Cartia XT SR 24 hour 120. Is... 1 reply
I was taking Diltiazem 120 24 HR. I just filled my prescription at Safeway and they gave me Cartia XT SR 24 hour 120. Is... 1 reply
Diltiazem xt 180 mg
Has anyone had the reddening of the skin due to peripheral vaso dilation, if so when med. Was discontinued did it subsid... 5 replies
Has anyone had the reddening of the skin due to peripheral vaso dilation, if so when med. Was discontinued did it subsid... 5 replies
Diltiazem Hydrochloride Extended Release Capsules
Not sure what they are for. ## How long does it take for diltia XT 180mg to leave your system and side effects to subsid... 8 replies
Not sure what they are for. ## How long does it take for diltia XT 180mg to leave your system and side effects to subsid... 8 replies
extended release capsules diltiazem 240 mg
Is it normal to have 4 little white pills inside the capsules? When my friend was taking her meds she found little white... 1 reply
Is it normal to have 4 little white pills inside the capsules? When my friend was taking her meds she found little white... 1 reply
Compare Diltiazem ER 180mg XT cap to two Diltiazem 90mg tablets @ 12hr intervals.
My new pharmacy plan puts my Diltiazem ER 180mg XT capsule into a Tier 3 costing me $60 for a 90 day supply. A tier 1 or... 1 reply
My new pharmacy plan puts my Diltiazem ER 180mg XT capsule into a Tier 3 costing me $60 for a 90 day supply. A tier 1 or... 1 reply
Diltiazem hcl sa caps 180mg vs Carti XT caps 180mg
I use to take Cardizem cd 180mg. I have lots of trouble with generics. I was switched to Diltiazem hcl sa caps some time... 2 replies
I use to take Cardizem cd 180mg. I have lots of trouble with generics. I was switched to Diltiazem hcl sa caps some time... 2 replies
What Is The Difference Between Diltiazem Xt And Cd
My prior prescription said I could take without regard to food. The new one said take on an empty stomach. Both are 180 ... 2 replies
My prior prescription said I could take without regard to food. The new one said take on an empty stomach. Both are 180 ... 2 replies
Diltiazem CD, XR & ER
What is the difference between diltiazem CD, diltiazem XR and diltiazem ER? Can you refer me to a website where I could ... 76 replies
What is the difference between diltiazem CD, diltiazem XR and diltiazem ER? Can you refer me to a website where I could ... 76 replies
diltiazem cd er
I have been told by my Neurologist that this drug Diltiazem cd can cause Parkinson like symptoms in a small group of peo... 8 replies
I have been told by my Neurologist that this drug Diltiazem cd can cause Parkinson like symptoms in a small group of peo... 8 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.