63629-1007 : Cefaclor 250 mg Oral Capsule
| NDC: | 63629-1007 |
| Labeler: | Bryant Ranch Prepack |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Cefaclor Cefaclor |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA065146 |
| Rev. Date: |
Appearance:
| Markings: | KRC |
| Shapes: |
Capsule |
| Colors: |
 Pink / Pink /
 Blue Blue |
| Size (mm): | 18 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
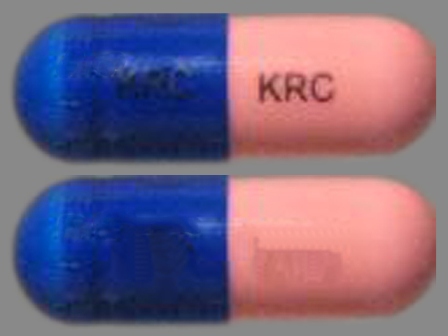
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 63629-1007-3: 30 CAPSULE IN 1 BOTTLE (63629‑1007‑3)
Active Ingredients:
- Cefaclor
Dosage Strength:
- 250 mg
Inactive Ingredients:
- Magnesium Stearate
- Sodium Starch Glycolate Type a Potato
- Lactose Monohydrate
- Talc
- Gelatin, Unspecified
- Titanium Dioxide
- Fd&c Blue No. 1
- Fd&c Red No. 3
- Shellac
- Ammonia
- Potassium Hydroxide
- Ferrosoferric Oxide
- Alcohol
- Isopropyl Alcohol
- Tert-butyl Alcohol
- Propylene Glycol /
Pharmaceutical Classes:
- Cephalosporin Antibacterial [EPC]
- Cephalosporins [CS]
Related Products:
Based on records with the same trade name.- 63629-1008 Cefaclor 500 mg Oral Capsule by Bryant Ranch Prepack
- 63629-1305 Cefaclor 250 mg Oral Capsule by Bryant Ranch Prepack
- 63629-1320 Cefaclor 250 mg Oral Capsule by Bryant Ranch Prepack
- 0093-1087 Cefaclor 500 mg 12 Hr Extended Release Tablet by Teva Pharmaceuticals USA Inc
- 0143-9985 Cefaclor 250 mg Oral Capsule by West-ward Pharmaceutical Corp
- 0143-9986 Cefaclor 500 mg Oral Capsule by West-ward Pharmaceutical Corp
- 13551-125 Cefaclor 125 mg/5ml Oral for Suspension by Fsc Laboratories, Inc.
- 13551-250 Cefaclor 250 mg/5ml Oral for Suspension by Fsc Laboratories, Inc.
- 13551-375 Cefaclor 375 mg/5ml Oral for Suspension by Fsc Laboratories, Inc.
- 16571-070 Cefaclor 125 mg/5ml Oral Suspension by Pack Pharmaceuticals, LLC
- 16571-071 Cefaclor 250 mg/5ml Oral Suspension by Pack Pharmaceuticals, LLC
- 16571-072 Cefaclor 375 mg/5ml Oral Suspension by Pack Pharmaceuticals, LLC
- 21695-782 Cefaclor 250 mg/5ml Oral Suspension by Rebel Distributors Corp
- 23594-125 Cefaclor 125 mg/5ml Oral for Suspension by Zylera Pharmaceuticals, LLC
- 23594-250 Cefaclor 250 mg/5ml Oral for Suspension by Zylera Pharmaceuticals, LLC
- 23594-375 Cefaclor 375 mg/5ml Oral for Suspension by Zylera Pharmaceuticals, LLC
- 43063-194 Cefaclor 500 mg Oral Capsule by Pd-rx Pharmaceuticals, Inc.
- 47781-266 Cefaclor 250 mg Oral Capsule by Alvogen, Inc.
- 47781-267 Cefaclor 500 mg Oral Capsule by Alvogen, Inc.
- 50090-0518 Cefaclor 500 mg Oral Capsule by A-s Medication Solutions
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 63629-1006Next: 63629-1008 >
Related Discussions:
Cefaclor Uses and Side Effects
What are the indications and side effects of Cefaclor? ## Cefaclor is a second-generation cephalosporin antibiotic used ... 1 reply
What are the indications and side effects of Cefaclor? ## Cefaclor is a second-generation cephalosporin antibiotic used ... 1 reply
cefaclor 500 capsule wrd
capsule ## Please see the page Drugs/Cefaclor.asp for more information about this medicine. Thank You!... 1 reply
capsule ## Please see the page Drugs/Cefaclor.asp for more information about this medicine. Thank You!... 1 reply
Ranbaxy Cefaclor MR
upper respitory tract infection...
upper respitory tract infection...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.