63459-225 : Nuvigil 250 mg Oral Tablet
| NDC: | 63459-225 |
| Labeler: | Cephalon, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Nuvigil Nuvigil |
| Dosage Form: | Oral Tablet |
| Application #: | NDA021875 |
| Rev. Date: | |
| CSA Schedule: | CIV (US) [1] |
[1] Schedule IV Controlled Substance: Low potential for abuse relative to substances in Schedule III. Examples include Alprazolam (Xanax), Diazepam (Valium), Carisoprodol (Soma), Clonazepam (Klonopin), Lorazepam (Ativan), Clorazepate (Tranxene), Midazolam (Versed), Temazepam (Restoril), and Triazolam (Halcion).. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | C;225 |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 16 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
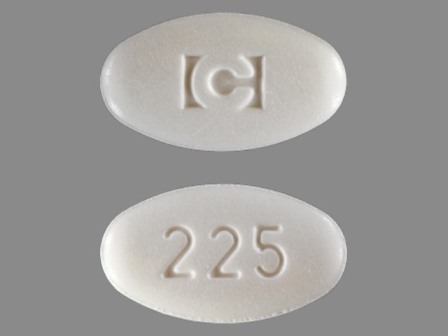
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 63459-225-30: 30 TABLET IN 1 BOTTLE (63459‑225‑30)
- 63459-225-35: 5 BLISTER PACK IN 1 CARTON (63459‑225‑35) > 7 TABLET IN 1 BLISTER PACK (63459‑225‑07)
Active Ingredients:
- Armodafinil
Dosage Strength:
- 250 mg
Inactive Ingredients:
- Croscarmellose Sodium
- Lactose Monohydrate
- Magnesium Stearate
- Cellulose, Microcrystalline
- Starch, Corn
- Povidones
Related Products:
Based on records with the same trade name.- 63459-205 Nuvigil 50 mg Oral Tablet by Cephalon, Inc.
- 63459-215 Nuvigil 150 mg Oral Tablet by Cephalon, Inc.
- 63459-220 Nuvigil 200 mg/1 Oral Tablet by Cephalon, Inc.
- 0179-0080 Nuvigil 250 mg Oral Tablet by Kaiser Foundation Hospitals
- 0179-0082 Nuvigil 50 mg Oral Tablet by Kaiser Foundation Hospitals
- 16590-345 Nuvigil 150 mg Oral Tablet by Stat Rx USA LLC
- 16590-394 Nuvigil 250 mg Oral Tablet by Stat Rx USA LLC
- 35356-588 Nuvigil 250 mg Oral Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 35356-589 Nuvigil 150 mg Oral Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 42254-014 Nuvigil 50 mg Oral Tablet by Rebel Distributors Corp
- 54569-6244 Nuvigil 150 mg Oral Tablet by A-s Medication Solutions LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 63459-220Next: 63459-303 >
Related Discussions:
Armodafinil prescription brands in U.S. comparison?
There are often generic brands coming and going on the market. So, I am asking peoples experience with current generics ...
There are often generic brands coming and going on the market. So, I am asking peoples experience with current generics ...
nuvigil side effects
I'm having severe side effects from nuvigil. Muscle and joint pain with numbness and tingling down my arms. ## Nuvig... 214 replies
I'm having severe side effects from nuvigil. Muscle and joint pain with numbness and tingling down my arms. ## Nuvig... 214 replies
Nuvigil for treatment resistant depression
Nuvigil (armodafinil) saved my life. I've had treatment resistant major depression for over 20 years. Tried so many ... 31 replies
Nuvigil (armodafinil) saved my life. I've had treatment resistant major depression for over 20 years. Tried so many ... 31 replies
Nuvigil Doses
can anybody tell me if they are prescribed more than 250mg per day for nuvigil. I take a 250 mg tablet at 8 a.m. and fee... 27 replies
can anybody tell me if they are prescribed more than 250mg per day for nuvigil. I take a 250 mg tablet at 8 a.m. and fee... 27 replies
Nuvigil Help / Severe opiate Addict
I am fresh off a 3 year opiate ride and to be quite honest I am the most weak and tired than I have ever been. The anxie... 26 replies
I am fresh off a 3 year opiate ride and to be quite honest I am the most weak and tired than I have ever been. The anxie... 26 replies
Nuvigil Crash and effects
I have been taking Nuvigil for a week to treat shift work sleep disorder. The first two times didnt have a huge effect o... 16 replies
I have been taking Nuvigil for a week to treat shift work sleep disorder. The first two times didnt have a huge effect o... 16 replies
Nuvigil 250 mg Patient Assistance
My insurance will no longer pay for my Nuvigil 250mg. It costs up to $1200. Month. They use to to pay for it. They will ... 5 replies
My insurance will no longer pay for my Nuvigil 250mg. It costs up to $1200. Month. They use to to pay for it. They will ... 5 replies
Nuvigil / Methadone
My husband has Narcolepsy and has started taking Nuvigil. He has been on Methadone for almost two months now.H e complai... 4 replies
My husband has Narcolepsy and has started taking Nuvigil. He has been on Methadone for almost two months now.H e complai... 4 replies
Nuvigil and hormonal changes?
I've been taking Nuvigil for years, and never had any real issues with side effects, but I did a bit of research on ... 4 replies
I've been taking Nuvigil for years, and never had any real issues with side effects, but I did a bit of research on ... 4 replies
Nuvigil side effects bloody stool
Started taking nuvigil 250 mg 2 weeks ago. Today noticed bloody stool is this a common side effect? Can't find any i... 4 replies
Started taking nuvigil 250 mg 2 weeks ago. Today noticed bloody stool is this a common side effect? Can't find any i... 4 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.