63395-201 : Evoxac 30 mg Oral Capsule
| NDC: | 63395-201 |
| Labeler: | Daiichi Sankyo Pharma Development |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Evoxac Evoxac |
| Dosage Form: | Oral Capsule |
| Application #: | NDA020989 |
| Rev. Date: |
Appearance:
| Markings: | EVOXAC;30;mg |
| Shapes: |
Capsule |
| Colors: |
 White White |
| Size (mm): | 15 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
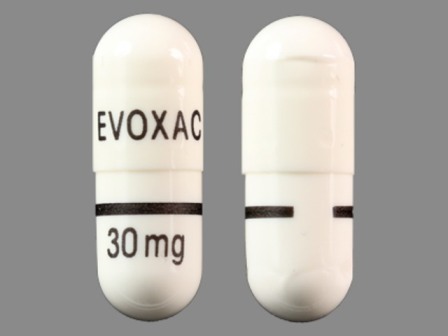
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 63395-201-13: 100 CAPSULE IN 1 BOTTLE, PLASTIC (63395‑201‑13)
Active Ingredients:
- Cevimeline Hydrochloride
Dosage Strength:
- 30 mg
Pharmaceutical Classes:
- Cholinergic Muscarinic Agonists [MoA]
- Cholinergic Receptor Agonist [EPC]
Related Products:
Based on records with the same trade name.- 0713-0883 Evoxac 30 mg Oral Capsule by Cosette Pharmaceuticals, Inc.
- 16590-859 Evoxac 30 mg Oral Capsule by Stat Rx USA LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 63388-999Next: 63398-1072 >
Related Discussions:
Evoxac cevimeline
I would like to know more about Evoxac Cevimeline 30 mg please. ## This medicine is a cholinergic agent used to treat dr... 2 replies
I would like to know more about Evoxac Cevimeline 30 mg please. ## This medicine is a cholinergic agent used to treat dr... 2 replies
cevimeline cap 30mg
i think i am having a side affect from the medicine... how long does it take to get out of your system ## Hello, Cathy! ... 1 reply
i think i am having a side affect from the medicine... how long does it take to get out of your system ## Hello, Cathy! ... 1 reply
side affects of evoxac
i have been takingg 30mg of Evoxac three times a day for about the last month for sjogrens disease.for the last week or ... 2 replies
i have been takingg 30mg of Evoxac three times a day for about the last month for sjogrens disease.for the last week or ... 2 replies
My new internist is against continued use of Evoxac!
My new internist wants me to discontinue use of evoxac to control my very dry mouth/esophagus/etc. She says that it has ... 2 replies
My new internist wants me to discontinue use of evoxac to control my very dry mouth/esophagus/etc. She says that it has ... 2 replies
Evoxac and possible heart problems?
Have you heard of this medication causing intermittent 2nd degree heart block or other rhythm problems?...
Have you heard of this medication causing intermittent 2nd degree heart block or other rhythm problems?...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.