61958-1004 : Ranexa 1000 mg 12 Hr Extended Release Tablet
| NDC: | 61958-1004 |
| Labeler: | Gilead Sciences, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Ranexa Ranexa |
| Dosage Form: | Oral Tablet, Film Coated, Extended Release |
| Application #: | NDA021526 |
| Rev. Date: |
Appearance:
| Markings: | GSI1000 |
| Shapes: |
Oval |
| Colors: |
 Yellow Yellow |
| Size (mm): | 21 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
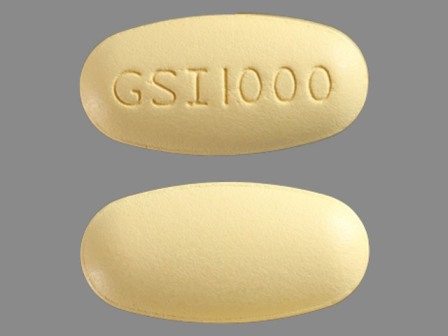
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 61958-1004-1: 60 TABLET, FILM COATED, EXTENDED RELEASE IN 1 BOTTLE, PLASTIC (61958‑1004‑1)
- 61958-1004-2: 14 TABLET, FILM COATED, EXTENDED RELEASE IN 1 BOTTLE, PLASTIC (61958‑1004‑2)
- 61958-1004-3: 14 TABLET, FILM COATED, EXTENDED RELEASE IN 1 BLISTER PACK (61958‑1004‑3)
Active Ingredients:
- Ranolazine
Dosage Strength:
- 1000 mg
Inactive Ingredients:
- Carnauba Wax
- Hypromelloses
- Magnesium Stearate
- Methacrylic Acid - Ethyl Acrylate Copolymer (1:1) Type a
- Cellulose, Microcrystalline
- Sodium Hydroxide
- Polyethylene Glycol 3350
- Titanium Dioxide
- Lactose Monohydrate
- Triacetin
- Ferric Oxide Yellow
- Hypromellose 2910 (6 Mpa.s)
- Water
Pharmaceutical Classes:
- Anti-anginal [EPC]
Related Products:
Based on records with the same trade name.- 61958-1001 Ranexa 500 mg 12 Hr Extended Release Tablet by Gilead Sciences, Inc.
- 61958-1002 Ranexa 1000 mg 12 Hr Extended Release Tablet by Gilead Sciences, Inc.
- 61958-1003 Ranexa 500 mg 12 Hr Extended Release Tablet by Gilead Sciences, Inc.
- 43353-880 Ranexa 500 mg Oral Tablet, Film Coated, Extended Release by Aphena Pharma Solutions - Tennessee, LLC
- 55154-4427 Ranexa 500 mg 12 Hr Extended Release Tablet by Cardinal Health
- 55154-4428 Ranexa 500 mg 12 Hr Extended Release Tablet by Cardinal Health
- 68151-5004 Ranexa 500 mg Oral Tablet, Film Coated, Extended Release by Carilion Materials Management
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 61958-1003Next: 61958-1101 >
Related Discussions:
Ranolazine 500mg
My mother is 65 years old, consuming Ranolazine - but i dont know what for? Please advise me. ## Ranolazine, the active ... 3 replies
My mother is 65 years old, consuming Ranolazine - but i dont know what for? Please advise me. ## Ranolazine, the active ... 3 replies
can Nikoran be used with ranolazine
In refractory angina after angioplasty what should be treatment guidelines ## I'm using nicorandil 10mg twice daily ... 1 reply
In refractory angina after angioplasty what should be treatment guidelines ## I'm using nicorandil 10mg twice daily ... 1 reply
Ranexa and Trazodone
I am supposed to start taking Ranexa for diffuse CAD. Intermittent angina. I take trazodone at night to help me sleep. T... 3 replies
I am supposed to start taking Ranexa for diffuse CAD. Intermittent angina. I take trazodone at night to help me sleep. T... 3 replies
Ranexa for Angina
My doctor gave me this because I have angina and when I lay down it hurts worse. What are the known side effects of this... 3 replies
My doctor gave me this because I have angina and when I lay down it hurts worse. What are the known side effects of this... 3 replies
ranexa 500mg
oval orange ## I feel drowsy, is this common? Ranexa has helped my angina. ## Yes, that can be a common side effect of t... 2 replies
oval orange ## I feel drowsy, is this common? Ranexa has helped my angina. ## Yes, that can be a common side effect of t... 2 replies
Ranexa problems
I have been on Ranexa for two days. Today while sitting at my computer I had a dizzy spell. I have no angina. My doctor ... 2 replies
I have been on Ranexa for two days. Today while sitting at my computer I had a dizzy spell. I have no angina. My doctor ... 2 replies
Ranexa and suggestions to help with constipation side effect
Any ideas on help for constipation side effect from the drug Ranexa? ## Hello, Nancy! How are you? A fiber supplement is... 2 replies
Any ideas on help for constipation side effect from the drug Ranexa? ## Hello, Nancy! How are you? A fiber supplement is... 2 replies
Ranexa in a sensitive system
I was recently given 500mg time-released caps of Ranexa after a heart cath, with blocked/failed stents but blood has re-... 1 reply
I was recently given 500mg time-released caps of Ranexa after a heart cath, with blocked/failed stents but blood has re-... 1 reply
Ranexa - can't sleep
I am on Ranexa for just 10 days 500 mg twice daily and each night I wake up at 2 or 3 am and cannot fall back asleep til... 1 reply
I am on Ranexa for just 10 days 500 mg twice daily and each night I wake up at 2 or 3 am and cannot fall back asleep til... 1 reply
Ranexa 500mg to 1000mg
I am on 500mg of Ranexa and my doctor has increased it to 1000mg. What difference will or could I feel? I have been exha... 1 reply
I am on 500mg of Ranexa and my doctor has increased it to 1000mg. What difference will or could I feel? I have been exha... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.