59762-0029 : Diclofenac Sodium (Enteric Coated Core) 75 mg / Misoprostol (Non-enteric Coated Mantle) 200 Mcg Oral Tablet
| NDC: | 59762-0029 |
| Labeler: | Greenstone LLC |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Diclofenac Sodium and Misoprostol Diclofenac Sodium and Misoprostol |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA020607 |
| Rev. Date: |
Appearance:
| Markings: | 75;G;0029 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 11 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
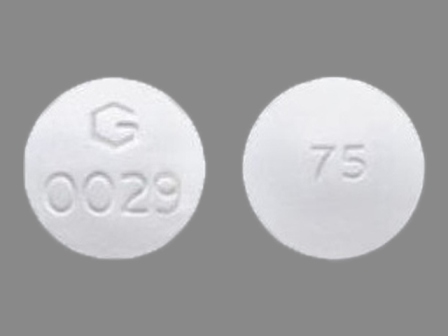
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 59762-0029-1: 60 TABLET, FILM COATED IN 1 BOTTLE (59762‑0029‑1)
- 59762-0029-3: 250 TABLET, FILM COATED IN 1 BOTTLE (59762‑0029‑3)
Active Ingredients:
- Diclofenac Sodium
- Misoprostol
Dosage Strength:
- 75 mg
- 200 ug/1
Inactive Ingredients:
- Silicon Dioxide
- Crospovidone
- Hydrogenated Castor Oil
- Hypromelloses
- Lactose
- Magnesium Stearate
- Cellulose, Microcrystalline
- Povidone K30
- Sodium Hydroxide
- Starch, Corn
- Talc
- Triethyl Citrate
Pharmaceutical Classes:
- Cyclooxygenase Inhibitors [MoA]
- Decreased Prostaglandin Production [PE]
- Nonsteroidal Anti-inflammatory Compounds [Chemical/Ingredient]
- Nonsteroidal Anti-inflammatory Drug [EPC]
- Prostaglandin E1 Analog [EPC]
- Prostaglandins E
- Synthetic [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 59762-0028 Diclofenac Sodium (Enteric Coated Core) 50 mg / Misoprostol (Non-enteric Coated Mantle) 200 Mcg Oral Tablet by Greenstone LLC
- 0591-0397 Diclofenac Sodium (Enteric Coated Core) 50 mg / Misoprostol (Non-enteric Coated Mantle) 200 Mcg Oral Tablet by Watson Laboratories, Inc.
- 0591-0398 Diclofenac Sodium (Enteric Coated Core) 75 mg / Misoprostol (Non-enteric Coated Mantle) 200 Mcg Oral Tablet by Watson Laboratories, Inc.
- 10544-949 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Blenheim Pharmacal, Inc.
- 42291-232 Diclofenac Sodium (Enteric Coated Core) 50 mg / Misoprostol (Non-enteric Coated Mantle) 200 Mcg Oral Tablet by Avkare, Inc.
- 42291-233 Diclofenac Sodium (Enteric Coated Core) 75 mg / Misoprostol (Non-enteric Coated Mantle) 200 Mcg Oral Tablet by Avkare, Inc.
- 50090-4618 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by A-s Medication Solutions
- 55648-215 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Wockhardt Limited
- 55648-217 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Wockhardt Limited
- 61919-094 Diclofenac Sodium and Misoprostol Oral Tablet, Film Coated by Direct Rx
- 63629-4970 Diclofenac Sodium and Misoprostol (Diclofenac Sodium 75 mg / Misoprostol 200 Ug) by Bryant Ranch Prepack
- 63629-7034 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Bryant Ranch Prepack
- 64679-215 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Wockhardt USA LLC.
- 64679-217 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Wockhardt USA LLC.
- 65162-436 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Amneal Pharmaceuticals, LLC
- 65162-438 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Amneal Pharmaceuticals, LLC
- 68001-231 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Bluepoint Laboratories
- 68001-232 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Bluepoint Laboratories
- 68084-978 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by American Health Packaging
- 68788-7103 Diclofenac Sodium and Misoprostol Oral Tablet, Delayed Release by Preferred Pharmaceuticals Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 59762-0028Next: 59762-0030 >
Related Discussions:
Diclofenac Sodium 75mg
I just took 2 diclofenac sodium 75mg at the same time. Since only one doesn't work at all. Could that hurt me? ## He... 5 replies
I just took 2 diclofenac sodium 75mg at the same time. Since only one doesn't work at all. Could that hurt me? ## He... 5 replies
diclofenac sodium 75 mg side effects
side effects for diclofenac sodium dr 75 mg tab ## From what I could gather, Diclofenac is known to cause the following ... 5 replies
side effects for diclofenac sodium dr 75 mg tab ## From what I could gather, Diclofenac is known to cause the following ... 5 replies
Diclofenac sodium use
I sprained my finger. Will this medicine help? ## Diclofenac Sodium is an anti-inflammatory, a generic for Voltaren. ## ... 4 replies
I sprained my finger. Will this medicine help? ## Diclofenac Sodium is an anti-inflammatory, a generic for Voltaren. ## ... 4 replies
diclofenac sodium, codeine phosphate tablet 50mg
I purchased diclofenac sodium & codeine phosphate tablets 50mg, but I cant find any images to identify it as such. I... 3 replies
I purchased diclofenac sodium & codeine phosphate tablets 50mg, but I cant find any images to identify it as such. I... 3 replies
Diclofenac sodium uses
## My husband has passed away and was a Dr. I have lots of this medication and would like to know what it is us for. ## ... 3 replies
## My husband has passed away and was a Dr. I have lots of this medication and would like to know what it is us for. ## ... 3 replies
diclofenac sodium 75 milligram
prescribed for a strained ligiment in my wrist.i have never taken before.i just want info. ## I just want info in genera... 3 replies
prescribed for a strained ligiment in my wrist.i have never taken before.i just want info. ## I just want info in genera... 3 replies
Diclofenac sodium paracetamol and chlorzoxazone tablets functions
for what treatment it is used ,WHAT are the side effects of these composition in a single tablet when consumed twice dai... 2 replies
for what treatment it is used ,WHAT are the side effects of these composition in a single tablet when consumed twice dai... 2 replies
diclofenac sodium dr 75 mg meloxicam 15 taken together side effects
my sister-in-law took both pills together and started stroke like symptoms. can they cause this if taken together? ## He... 1 reply
my sister-in-law took both pills together and started stroke like symptoms. can they cause this if taken together? ## He... 1 reply
Diclofenac Sodium Paracetamol And Chlorzoxazone
i want to know the need of the tablet of the following drug content: Diclofenac, Sodium Paracetamol And Chlorzoxazone ##... 1 reply
i want to know the need of the tablet of the following drug content: Diclofenac, Sodium Paracetamol And Chlorzoxazone ##... 1 reply
Diclofenac Sodium Ingredients
Does Diclofenac Sodium contain sulfa? ## Diclofenac Sodium is exactly that, a sodium salt, not a sulfa drug. As to the i... 1 reply
Does Diclofenac Sodium contain sulfa? ## Diclofenac Sodium is exactly that, a sodium salt, not a sulfa drug. As to the i... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.