55700-004 : Cyclobenzaprine Hydrochloride 5 mg Oral Tablet
| NDC: | 55700-004 |
| Labeler: | Lake Erie Medical Dba Quality Care Products LLC |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Cyclobenzaprine Hydrochloride Cyclobenzaprine Hydrochloride |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | ANDA077797 |
| Rev. Date: |
Appearance:
| Markings: | 2631;V |
| Shapes: |
Round |
| Colors: |
 Orange Orange |
| Size (mm): | 7 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
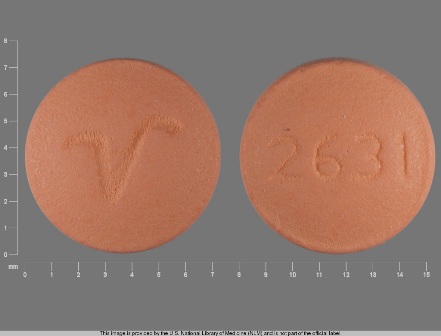
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 55700-004-01: 120 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55700‑004‑01)
- 55700-004-10: 10 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55700‑004‑10)
- 55700-004-12: 12 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55700‑004‑12)
- 55700-004-15: 15 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55700‑004‑15)
- 55700-004-20: 20 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55700‑004‑20)
- 55700-004-21: 21 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55700‑004‑21)
- 55700-004-30: 30 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55700‑004‑30)
- 55700-004-60: 60 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55700‑004‑60)
- 55700-004-90: 90 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55700‑004‑90)
Active Ingredients:
- Cyclobenzaprine Hydrochloride
Dosage Strength:
- 5 mg
Inactive Ingredients:
- Croscarmellose Sodium
- Fd&c Yellow No. 6
- Hypromelloses
- Lactose Monohydrate
- Magnesium Stearate
- Cellulose, Microcrystalline
- Polyethylene Glycols
- Titanium Dioxide
- Fd&c Red No. 40
Pharmaceutical Classes:
- Centrally-mediated Muscle Relaxation [PE]
- Muscle Relaxant [EPC]
Related Products:
Based on records with the same trade name.- 55700-229 Cyclobenzaprine Hydrochloride 5 mg Oral Tablet, Film Coated by Lake Erie Medical Dba Quality Care Products LLC
- 55700-239 Cyclobenzaprine Hydrochloride 7.5 mg Oral Tablet, Film Coated by Lake Erie Medical Dba Quality Care Products LLC
- 55700-311 Cyclobenzaprine Hydrochloride 10 mg Oral Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 55700-459 Cyclobenzaprine Hydrochloride 10 mg Oral Tablet, Film Coated by Lake Erie Medical Dba Quality Care Products LLC
- 55700-482 Cyclobenzaprine Hydrochloride 5 mg Oral Tablet, Film Coated by Lake Erie Medical Dba Quality Care Products LLC
- 55700-527 Cyclobenzaprine Hydrochloride 5 mg Oral Tablet, Film Coated by Lake Erie Medical Dba Quality Care Products LLC
- 55700-599 Cyclobenzaprine Hydrochloride 10 mg Oral Tablet, Film Coated by Lake Erie Medical Dba Quality Care Products LLC
- 55700-696 Cyclobenzaprine Hydrochloride 5 mg Oral Tablet, Film Coated by Quality Care Products, LLC
- 55700-762 Cyclobenzaprine Hydrochloride 7.5 mg Oral Tablet, Film Coated by Quality Care Products, LLC
- 55700-857 Cyclobenzaprine Hydrochloride 15 mg Oral Capsule, Extended Release by Quality Care Products, LLC
- 55700-858 Cyclobenzaprine Hydrochloride 30 mg Oral Capsule, Extended Release by Quality Care Products, LLC
- 55700-925 Cyclobenzaprine Hydrochloride 10 mg Oral Tablet, Film Coated by Quality Care Products, LLC
- 55700-926 Cyclobenzaprine Hydrochloride 5 mg Oral Tablet, Film Coated by Quality Care Products, LLC
- 55700-981 Cyclobenzaprine Hydrochloride 15 mg Oral Capsule, Extended Release by Quality Care Products, LLC
- 35356-717 Cyclobenzaprine Hydrochloride 10 mg Oral Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 35356-847 Cyclobenzaprine Hydrochloride 5 mg Oral Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 0093-1920 Cyclobenzaprine Hydrochloride 15 mg Oral Capsule, Extended Release by Teva Pharmaceuticals USA, Inc.
- 0093-1921 Cyclobenzaprine Hydrochloride 30 mg Oral Capsule, Extended Release by Teva Pharmaceuticals USA, Inc.
- 0093-3420 Cyclobenzaprine Hydrochloride 5 mg Oral Tablet, Film Coated by Teva Pharmaceuticals USA, Inc.
- 0093-3421 Cyclobenzaprine Hydrochloride 7.5 mg Oral Tablet, Film Coated by Teva Pharmaceuticals USA, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 55700-003Next: 55700-005 >
Related Discussions:
Cyclobenzaprine 5mg Shelf Life
what is the shelf life of cyclobenzapine 5 mg? ## Hi cindirs, According to Rising Pharmaceuticals, a manufacturer of Cyc... 7 replies
what is the shelf life of cyclobenzapine 5 mg? ## Hi cindirs, According to Rising Pharmaceuticals, a manufacturer of Cyc... 7 replies
Cyclobenzaprine 10mg and heart health
If this drug cyclobenzaprine (10mg) is a muscle relaxer and the heart is a muscle, if I'm taking 1 at a time, is the... 6 replies
If this drug cyclobenzaprine (10mg) is a muscle relaxer and the heart is a muscle, if I'm taking 1 at a time, is the... 6 replies
Cyclobenzaprine side effects
I am on this medication, taking 5 mg once a day at bedtime. I feel tired all the time and have very little energy. Shoul... 5 replies
I am on this medication, taking 5 mg once a day at bedtime. I feel tired all the time and have very little energy. Shoul... 5 replies
Cyclobenzaprine 10mg Tablet
My gp prescribed me 50 mgs amitrypteline one at night is this a muscle relaxant ! only the only time i havent got this a... 5 replies
My gp prescribed me 50 mgs amitrypteline one at night is this a muscle relaxant ! only the only time i havent got this a... 5 replies
Cyclobenzaprine 10mg Tab M 751 Round Peach Color
can you take a cyclobenzaprine now and later today take a tumornal? ## I'm sorry, but I can't find any informati... 4 replies
can you take a cyclobenzaprine now and later today take a tumornal? ## I'm sorry, but I can't find any informati... 4 replies
Cyclobenzaprine 10mg png
M on front, 751 on back, round peachy-orange color ## Yes, this is a 10mg Cyclobenzaprine tablet, the active ingredient ... 4 replies
M on front, 751 on back, round peachy-orange color ## Yes, this is a 10mg Cyclobenzaprine tablet, the active ingredient ... 4 replies
cyclobenzaprine 10mg tab
small round pill, tan in color..there is an M on one side and number 751 on the other. ## would like to know what this i... 4 replies
small round pill, tan in color..there is an M on one side and number 751 on the other. ## would like to know what this i... 4 replies
Cyclobenzaprine & Cyclobenzapar
Is there a difference between Cyclobenzaprine 5 mg tablet and Cyclobenzapar 5 mg tablet, My prescription was written for... 3 replies
Is there a difference between Cyclobenzaprine 5 mg tablet and Cyclobenzapar 5 mg tablet, My prescription was written for... 3 replies
Cyclobenzaprine Tab 5 Mg muscle relaxer
Are these pills similar/same: cyclobenzapr 5 mg, (blue) and cyclobenzaprine 5mg (orange) but smaller: generic for Flexer... 3 replies
Are these pills similar/same: cyclobenzapr 5 mg, (blue) and cyclobenzaprine 5mg (orange) but smaller: generic for Flexer... 3 replies
Cyclobenzaprine a opiate?
My boy friend is coming off of pain killers , higher doses other known as oxys if he takes these 10mg yellow pills will ... 3 replies
My boy friend is coming off of pain killers , higher doses other known as oxys if he takes these 10mg yellow pills will ... 3 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.