55289-612 : Zanaflex 4 mg Oral Tablet
| NDC: | 55289-612 |
| Labeler: | Pd-rx Pharmaceuticals, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Zanaflex Zanaflex |
| Dosage Form: | Oral Tablet |
| Application #: | NDA020397 |
| Rev. Date: |
Appearance:
| Markings: | A594 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 9 |
| Segments: * | 4 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 4 indicates a scored pill which can be broken into 4 equal pieces. | |
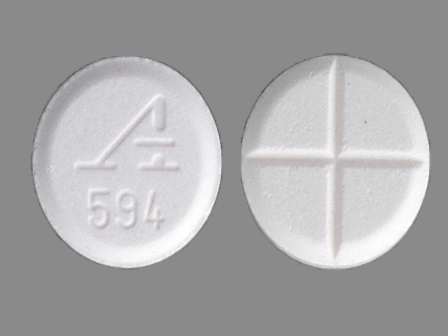
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 55289-612-10: 10 TABLET IN 1 BOTTLE, PLASTIC (55289‑612‑10)
- 55289-612-20: 20 TABLET IN 1 BOTTLE, PLASTIC (55289‑612‑20)
Active Ingredients:
- Tizanidine Hydrochloride
Dosage Strength:
- 4 mg
Inactive Ingredients:
- Silicon Dioxide
- Stearic Acid
- Cellulose, Microcrystalline
- Anhydrous Lactose
Pharmaceutical Classes:
- Adrenergic alpha2-Agonists [MoA]
- Central alpha-2 Adrenergic Agonist [EPC]
Related Products:
Based on records with the same trade name.- 10144-594 Zanaflex 4 mg Oral Tablet by Acorda Therapeutics, Inc.
- 10144-602 Zanaflex 2 mg Oral Capsule by Acorda Therapeutics, Inc.
- 10144-604 Zanaflex 4 mg Oral Capsule by Acorda Therapeutics, Inc.
- 10144-606 Zanaflex 6 mg Oral Capsule by Acorda Therapeutics, Inc.
- 16590-756 Zanaflex 6 mg Oral Capsule by Stat Rx USA LLC
- 16590-871 Zanaflex 4 mg Oral Capsule by Stat Rx USA LLC
- 21695-373 Zanaflex 4 mg Oral Capsule by Rebel Distributors Corp
- 35356-341 Zanaflex 2 mg Oral Capsule by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
- 35356-342 Zanaflex 4 mg Oral Capsule by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
- 35356-446 Zanaflex 6 mg Oral Capsule by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
- 70515-594 Zanaflex 4 mg Oral Tablet by Covis Pharma
- 70515-602 Zanaflex 2 mg Oral Capsule by Covis Pharma
- 70515-604 Zanaflex 4 mg Oral Capsule by Covis Pharma
- 70515-606 Zanaflex 6 mg Oral Capsule by Covis Pharma
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 55289-611Next: 55289-613 >
Related Discussions:
tizanidine 4mg or soma 350mg stronger
Was taking soma till my insurance put me on tizanadine 4mg once a day which does nothing is that a comparison choice? ##... 35 replies
Was taking soma till my insurance put me on tizanadine 4mg once a day which does nothing is that a comparison choice? ##... 35 replies
Tizanidine Medication
I have had hallucinations and terrible, very realistic nightmares. I finally figured out that it was only after I took m... 11 replies
I have had hallucinations and terrible, very realistic nightmares. I finally figured out that it was only after I took m... 11 replies
Tizanidine feels toxic, like I need to clean it out!
Basically have been trying to manage shoulder or possibly neck tensing issue for about 4-5 months now. I also try to rem... 8 replies
Basically have been trying to manage shoulder or possibly neck tensing issue for about 4-5 months now. I also try to rem... 8 replies
TIZANIDINE HCL 4 MG TABLET
round whit pill, with e 44 on on side and a criss cross on the other. This is a SKELETAL MUSCLE RELAXANT ## This medicat... 6 replies
round whit pill, with e 44 on on side and a criss cross on the other. This is a SKELETAL MUSCLE RELAXANT ## This medicat... 6 replies
tizanidine a narcotic
Is tizanidine considered a narcotic? Thanks ## Hello, Donald! How are you? No, Tizanidine is a muscle relaxant. It may c... 3 replies
Is tizanidine considered a narcotic? Thanks ## Hello, Donald! How are you? No, Tizanidine is a muscle relaxant. It may c... 3 replies
Tizanidine 4mg drowsiness and dizziness
I have been on tizanidine, for about two weeks give or take. I was wandering if anyone could tell me if the morning dose... 3 replies
I have been on tizanidine, for about two weeks give or take. I was wandering if anyone could tell me if the morning dose... 3 replies
Tizanidine-HCL drug info
## I was prescribed tizanidine for muscle pain will this medication trigger my anxiety ## I wouldn't think it would.... 3 replies
## I was prescribed tizanidine for muscle pain will this medication trigger my anxiety ## I wouldn't think it would.... 3 replies
Tizanidine Abnormal Dreams Hallicinations
I have been taking Tizanidine 12mg at night and 4 mg in the morning for over 3 months and have been experiencing some al... 3 replies
I have been taking Tizanidine 12mg at night and 4 mg in the morning for over 3 months and have been experiencing some al... 3 replies
tizanidine hcl 4mgcom
football shape white ri80 and cress cross on tablet ## i dont know if this my musal relaxer or not i think it is but i a... 2 replies
football shape white ri80 and cress cross on tablet ## i dont know if this my musal relaxer or not i think it is but i a... 2 replies
tizanidine hcl 4 mg tablet car
round white pill with the number 503 on one side and an x marking on the otherside ## is tizanidine hcl considered a nar... 2 replies
round white pill with the number 503 on one side and an x marking on the otherside ## is tizanidine hcl considered a nar... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.