55154-3938 : Zyvox 600 mg Oral Tablet
| NDC: | 55154-3938 |
| Labeler: | Cardinal Health |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Zyvox Zyvox |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA021130 |
| Rev. Date: |
Appearance:
| Markings: | ZYVOX;600mg |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 18 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
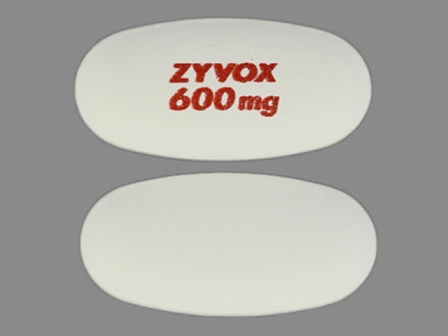
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 55154-3938-0: 10 BLISTER PACK IN 1 BAG (55154‑3938‑0) > 1 TABLET, FILM COATED IN 1 BLISTER PACK
Active Ingredients:
- Linezolid
Dosage Strength:
- 600 mg
Inactive Ingredients:
- Starch, Corn
- Cellulose, Microcrystalline
- Hydroxypropyl Cellulose
- Sodium Starch Glycolate Type a Potato
- Magnesium Stearate
- Hypromelloses
- Polyethylene Glycol
- Titanium Dioxide
- Carnauba Wax
Pharmaceutical Classes:
- Oxazolidinone Antibacterial [EPC]
- Oxazolidinones [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 0009-4992 Zyvox 600 mg/300ml Intravenous Injection, Solution by Pharmacia and Upjohn Company
- 0009-5134 Zyvox 400 mg Oral Tablet by Pharmacia and Upjohn Company
- 0009-5135 Zyvox 600 mg Oral Tablet by Pharmacia and Upjohn Company
- 0009-5136 Zyvox 100 mg/5ml Oral Suspension by Pharmacia and Upjohn Company
- 0009-5137 Zyvox 200 mg/100ml Intravenous Injection, Solution by Pharmacia and Upjohn Company
- 0009-5138 Zyvox 600 mg Oral Tablet, Film Coated by Pharmacia and Upjohn Company
- 0009-5139 Zyvox 400 mg/200ml Intravenous Injection, Solution by Pharmacia and Upjohn Company
- 0009-5140 Zyvox 600 mg/300ml Intravenous Injection, Solution by Pharmacia and Upjohn Company
- 0009-7807 Zyvox 600 mg/300ml Intravenous Injection, Solution by Pharmacia and Upjohn Company
- 21695-399 Zyvox 600 mg Oral Tablet by Rebel Distributors Corp
- 49349-751 Zyvox 600 mg Oral Tablet by Remedyrepack Inc.
- 55695-009 Zyvox 100 mg/5ml Oral Suspension by Department of State Health Services, Pharmacy Branch
- 66298-4992 Zyvox 600 mg/300ml Intravenous Injection, Solution by Fresenius Kabi Norge As
- 66298-5137 Zyvox 200 mg/100ml Intravenous Injection, Solution by Fresenius Kabi Norge As
- 66298-5140 Zyvox 600 mg/300ml Intravenous Injection, Solution by Fresenius Kabi Norge As
- 66298-7807 Zyvox 600 mg/300ml Intravenous Injection by Fresenius Kabi Norge As
- 70518-1226 Zyvox 600 mg Oral Tablet, Film Coated by Remedyrepack Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 55154-3935Next: 55154-3939 >
Related Discussions:
linezolid ip 600mg side effects
I am suffering from headaches and blurred vision even after completion of linezolid ip 600mg antibiotic tablets that I h...
I am suffering from headaches and blurred vision even after completion of linezolid ip 600mg antibiotic tablets that I h...
Cost of zyvox injection 200mg/100ml
I have 30 individual doses of this med that my son was receiving via picc line at home. Just trying to get an idea of th... 3 replies
I have 30 individual doses of this med that my son was receiving via picc line at home. Just trying to get an idea of th... 3 replies
Linezolid
Zyvox ## my tongue has turned black is this a reaction to combination of meds? will it go away there is no pain or taste... 1 reply
Zyvox ## my tongue has turned black is this a reaction to combination of meds? will it go away there is no pain or taste... 1 reply
Linezolid cmarc date and acceptance
whats the acceptance of drug in patient curity and cmarc data for dr prescription?...
whats the acceptance of drug in patient curity and cmarc data for dr prescription?...
can linezolid used for tinea infection
Can use this medicine for curing tinea infection...
Can use this medicine for curing tinea infection...
zyvox cost
doctor prescribed zyvox but insurance do not cover, med over $3000...
doctor prescribed zyvox but insurance do not cover, med over $3000...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.