54868-5983 : Exforge 10/320 (Amlodipine / Valsartan) Oral Tablet
| NDC: | 54868-5983 |
| Labeler: | Physicians Total Care, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Exforge Exforge |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA021990 |
| Rev. Date: |
Appearance:
| Markings: | NVR;LUF |
| Shapes: |
Oval |
| Colors: |
 Yellow Yellow |
| Size (mm): | 19 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
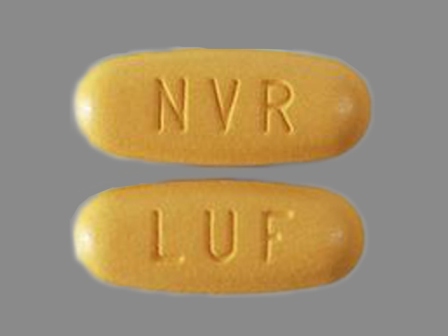
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 54868-5983-0: 30 TABLET, FILM COATED IN 1 BOTTLE (54868‑5983‑0)
- 54868-5983-1: 90 TABLET, FILM COATED IN 1 BOTTLE (54868‑5983‑1)
Active Ingredients:
- Amlodipine Besylate
- Valsartan
Dosage Strength:
- 10 mg
- 320 mg
Inactive Ingredients:
- Silicon Dioxide
- Crospovidone
- Hypromelloses
- Ferric Oxide Yellow
- Magnesium Stearate
- Cellulose, Microcrystalline
- Polyethylene Glycol
- Sodium Starch Glycolate Type a Potato
- Talc
- Titanium Dioxide
Pharmaceutical Classes:
- Calcium Channel Antagonists [MoA]
- Dihydropyridine Calcium Channel Blocker [EPC]
- Dihydropyridines [Chemical/Ingredient]
- Angiotensin 2 Receptor Antagonists [MoA]
- Angiotensin 2 Receptor Blocker [EPC]
Related Products:
Based on records with the same trade name.- 54868-5804 Exforge 10/160 Oral Tablet by Physicians Total Care, Inc.
- 54868-5996 Exforge 5/320 (Amlodipine / Valsartan) Oral Tablet by Physicians Total Care, Inc.
- 54868-5997 Exforge 5/160 (Amlodipine / Valsartan) Oral Tablet by Physicians Total Care, Inc.
- 0078-0488 Exforge 5/160 (Amlodipine / Valsartan) Oral Tablet by Novartis Pharmaceuticals Corporation
- 0078-0489 Exforge 10/160 Oral Tablet by Novartis Pharmaceuticals Corporation
- 0078-0490 Exforge 5/320 (Amlodipine / Valsartan) Oral Tablet by Novartis Pharmaceuticals Corporation
- 0078-0491 Exforge 10/320 (Amlodipine / Valsartan) Oral Tablet by Novartis Pharmaceuticals Corporation
- 52125-853 Exforge Oral Tablet, Film Coated by Remedyrepack Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 54868-5982Next: 54868-5984 >
Related Discussions:
amlodipine besylate is there any alternative
I need to view medication that are healthy alternative for Amlodipine ## What alternative medication if any that could b... 17 replies
I need to view medication that are healthy alternative for Amlodipine ## What alternative medication if any that could b... 17 replies
Amlodipine Besylate coated pill
I have been taking 10 mg ofr Amlodipine Besylate. My problem is that it tastes horrible. Are there any of these in the g... 13 replies
I have been taking 10 mg ofr Amlodipine Besylate. My problem is that it tastes horrible. Are there any of these in the g... 13 replies
amlodipine besylate 5 mg tamyl
i am looking for side effects of this drug ## Some side effects of the use of amlodipine may be: Very often: peripheral ... 8 replies
i am looking for side effects of this drug ## Some side effects of the use of amlodipine may be: Very often: peripheral ... 8 replies
amlodipine besylate 10 mg is this time released
is the medicine amlodipine besylate medicine time released. ## Hi Barbara, Usually if a medication is time-released it w... 7 replies
is the medicine amlodipine besylate medicine time released. ## Hi Barbara, Usually if a medication is time-released it w... 7 replies
Amlodipine Besylate and Losartan
Can these be taken together in the evening? And will it be effective for 24 hrs? I'm too sleepy during the day!! ## ... 6 replies
Can these be taken together in the evening? And will it be effective for 24 hrs? I'm too sleepy during the day!! ## ... 6 replies
amlodipine besylate 10mg
White, elongated octagon, AM10 ## I'd like to see an image of it ## There is no pill matching this description in th... 5 replies
White, elongated octagon, AM10 ## I'd like to see an image of it ## There is no pill matching this description in th... 5 replies
Amlodipine Besylate 10 Mg Tab Color
I am on 10 mg of Amlodipine Besylate. The pill is usually white in color. I pick up my perscription yesterday, open the ... 5 replies
I am on 10 mg of Amlodipine Besylate. The pill is usually white in color. I pick up my perscription yesterday, open the ... 5 replies
Amlodipine Besylate Ta AND LEMON JUICE
can drinking lemon juice reduce the effectivness of this pill. Warning on bottle says not to take grapefruit juice! ## N... 4 replies
can drinking lemon juice reduce the effectivness of this pill. Warning on bottle says not to take grapefruit juice! ## N... 4 replies
amlodipine besylate 5 mg tab
this is a white, octagonal whith G 1530 on the front and 5 on the back ## Just to confirm, I also located this pill to b... 4 replies
this is a white, octagonal whith G 1530 on the front and 5 on the back ## Just to confirm, I also located this pill to b... 4 replies
Amlodipine Besylate are all generics the same
Are all Generic created equally. I was taking the generic Amlodipine Besylate that was Blue with A9 on front an M on bac... 3 replies
Are all Generic created equally. I was taking the generic Amlodipine Besylate that was Blue with A9 on front an M on bac... 3 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.