54868-5534 : Dph Sodium 100 mg Extended Release Capsule
| NDC: | 54868-5534 |
| Labeler: | Physicians Total Care, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Phenytoin Sodium Phenytoin Sodium |
| Dosage Form: | Oral Capsule, Extended Release |
| Application #: | ANDA040684 |
| Rev. Date: |
Appearance:
| Markings: | TARO;PHN;100 |
| Shapes: |
Capsule |
| Colors: |
 Orange Orange |
| Size (mm): | 16 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
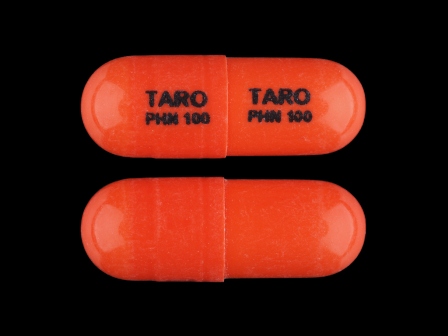
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 54868-5534-0: 90 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE (54868‑5534‑0)
- 54868-5534-1: 100 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE (54868‑5534‑1)
- 54868-5534-2: 30 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE (54868‑5534‑2)
- 54868-5534-3: 60 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE (54868‑5534‑3)
- 54868-5534-4: 120 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE (54868‑5534‑4)
Active Ingredients:
- Phenytoin Sodium
Dosage Strength:
- 100 mg
Inactive Ingredients:
- Sucrose
- Hypromelloses
- Lactose Monohydrate
- Magnesium Stearate
- Talc
- Benzyl Alcohol
- Butylparaben
- D&c Yellow No. 10
- Edetate Calcium Disodium
- Fd&c Red No. 3
- Gelatin
- Methylparaben
- Propylparaben
- Sodium Lauryl Sulfate
- Sodium Propionate
- Titanium Dioxide
Pharmaceutical Classes:
- Anti-epileptic Agent [EPC]
- Decreased Central Nervous System Disorganized Electrical Activity [PE]
- Cytochrome P450 1A2 Inducers [MoA]
- Cytochrome P450 2B6 Inducers [MoA]
- Cytochrome P450 2C8 Inducers [MoA]
- Cytochrome P450 2C19 Inducers [MoA]
- Cytochrome P450 2D6 Inducers [MoA]
- Cytochrome P450 3A Inducers [MoA]
- Cytochrome P450 2C9 Inducers [MoA]
Related Products:
Based on records with the same trade name.- 0378-1560 Dph Sodium 100 mg Extended Release Capsule by Mylan Pharmaceuticals Inc.
- 0404-9932 Phenytoin Sodium 50 mg/ml Intramuscular; Intravenous Injection by Henry Schein, Inc.
- 0404-9933 Phenytoin Sodium 250 mg/5ml Intramuscular; Intravenous Injection by Henry Schein, Inc.
- 0409-1317 Phenytoin Sodium 50 mg/ml Intramuscular; Intravenous Injection, Solution by Hospira, Inc.
- 0409-1844 Phenytoin Sodium 50 mg/ml Intramuscular; Intravenous Injection, Solution by Hospira, Inc.
- 0615-1343 Dph Sodium 100 mg Extended Release Capsule by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0615-8020 Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0641-0493 Phenytoin Sodium 50 mg/ml Intramuscular; Intravenous Injection by West-ward Pharmaceutical Corp.
- 0641-2555 Phenytoin Sodium 50 mg/ml Intramuscular; Intravenous Injection by West-ward Pharmaceutical Corp.
- 0641-6138 Phenytoin Sodium 50 mg/ml Intramuscular; Intravenous Injection by West-ward Pharmaceutical Corp.
- 0641-6139 Phenytoin Sodium 50 mg/ml Intramuscular; Intravenous Injection by West-ward Pharmaceutical Corp.
- 0832-0620 Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Upsher-smith Laboratories, Inc.
- 21695-167 Dph Sodium 100 mg Extended Release Capsule by Rebel Distributors Corp
- 29300-379 Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Unichem Pharmaceuticals (Usa), Inc.
- 39822-4050 Phenytoin Sodium 50 mg/ml Intramuscular; Intravenous Injection, Solution by X-gen Pharmaceuticals, Inc.
- 42192-614 Phenytoin Sodium 50 mg/ml Intramuscular; Intravenous Injection by Acella Pharmaceuticals, LLC
- 49999-574 Dph Sodium 100 mg Extended Release Capsule by Lake Erie Medical Dba Quality Care Products LLC
- 50090-1480 Phenytoin Sodium 100 mg Oral Capsule, Extended Release by A-s Medication Solutions
- 50090-2903 Phenytoin Sodium 100 mg Oral Capsule, Extended Release by A-s Medication Solutions
- 50090-3499 Phenytoin Sodium 100 mg Oral Capsule, Extended Release by A-s Medication Solutions
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 54868-5533Next: 54868-5538 >
Related Discussions:
Phenytoin Sodium 100 MG Ext Capsules
What are capsule color(s) for AMNEAL vs. color(s) for SUN…thx. ## There are several manufactured by ea... 2 replies
What are capsule color(s) for AMNEAL vs. color(s) for SUN…thx. ## There are several manufactured by ea... 2 replies
phenytoin sodium IP
disadvantages of phenytoin sodium IP ## Is toinex - 100 as effective as Dilantin? And, the Toinex I have received is a r... 2 replies
disadvantages of phenytoin sodium IP ## Is toinex - 100 as effective as Dilantin? And, the Toinex I have received is a r... 2 replies
phenytoin sod ext 100 mg casun
PHENYTION SOD EXT 100MG CASUN/ GERNERICK OF DALANTON WHITE CAPSULE/ FOR SEVERE HEAD CONCUSSION / HAVING BAD NECK PAINS,R... 5 replies
PHENYTION SOD EXT 100MG CASUN/ GERNERICK OF DALANTON WHITE CAPSULE/ FOR SEVERE HEAD CONCUSSION / HAVING BAD NECK PAINS,R... 5 replies
phenytoin ex 100mg side effects
IT IS A PURPLE AND WHITE CAPSULE EVERY TIME MY HUSBAND TAKE IT ABOUT 2 HRS LATTER HIS FACE BREAK OUT THEN IT PEELS IT IT... 5 replies
IT IS A PURPLE AND WHITE CAPSULE EVERY TIME MY HUSBAND TAKE IT ABOUT 2 HRS LATTER HIS FACE BREAK OUT THEN IT PEELS IT IT... 5 replies
phenytoin 420 - plain white gel capsule with imprint 420 twice, no other markings
My prescription drug was changed from phenytoin with the purple band to phenytoin plain white with the number 420 printe... 3 replies
My prescription drug was changed from phenytoin with the purple band to phenytoin plain white with the number 420 printe... 3 replies
phenytoin ex cap 100mg
do phenytoin ex contain nitrates such as nitroglycein ## My boyfriend has been prescribed Phenytoin ex 100 mg. 3 tabs b4... 3 replies
do phenytoin ex contain nitrates such as nitroglycein ## My boyfriend has been prescribed Phenytoin ex 100 mg. 3 tabs b4... 3 replies
Phenytoin Sod Ext 100 Mg Cap purple and white capsule with ip212 on it
I've been taking these for a while and recently stopped taking them for a month. I wanted to see the difference in m... 3 replies
I've been taking these for a while and recently stopped taking them for a month. I wanted to see the difference in m... 3 replies
PHENYTOIN SOD EXT 100MG
capsule,purple/white,1560,1540 ## Want to know about the 40 phenytoin SOD action, side effects, substitute, alternatie, ... 3 replies
capsule,purple/white,1560,1540 ## Want to know about the 40 phenytoin SOD action, side effects, substitute, alternatie, ... 3 replies
phenytoin I.P 100mg eptoin
My brother has tapeworm in his brain and prescribed for phenytoin eption is it right medicine?? Now a days he is felling... 2 replies
My brother has tapeworm in his brain and prescribed for phenytoin eption is it right medicine?? Now a days he is felling... 2 replies
Phenytoin Ex Caps 100 Mg for Severe diabetic neurology pain
Severe diabetic neurology pain in both legs. Leg cramps, restricted ability to walk and stand. ## This medicine combined... 2 replies
Severe diabetic neurology pain in both legs. Leg cramps, restricted ability to walk and stand. ## This medicine combined... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.